Solvent-assisted iron oxide nanoparticles for selective electrochemical detection of chlorogenic acid in real samples
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-09-16
DOI:10.1016/j.jtice.2025.106378
引用次数: 0
Abstract
Backgrounds
Iron oxide (α-Fe2O3, hematite) is a widely available, cost-effective, and highly stable p-type semiconductor with significant potential for various applications. Due to its excellent electrochemical properties, hematite has been extensively employed in detecting toxic pollutants. However, optimizing its synthesis conditions can enhance its electrocatalytic efficiency, making it a promising material for electrochemical sensing applications.
Methods
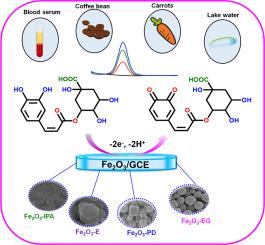
Hematite (α-Fe₂O₃) nanoparticles were synthesized using a solvothermal method by varying solvents. The structural and morphological properties were characterized using PXRD, FT-IR, BET, XPS, FE-SEM, and HR-TEM techniques. Electrochemical performance was evaluated using cyclic voltammetry (CV) and square wave voltammetry (SWV), while the analysis of electron transfer kinetics was conducted via electrochemical impedance spectroscopy (EIS). The Fe₂O₃-E (Iron oxide prepared using ethanol as solvent) modified GCE (glassy carbon electrode) sensor was then tested for chlorogenic acid (CGA) detection under optimized conditions.
Significance
The developed Fe₂O₃-E modified GCE sensor exhibited outstanding electrocatalytic activity for CGA detection, demonstrating a low detection limit (0.0066 µM) and a wide linear range (0.05–668 µM). It showed excellent selectivity despite potential interferences and maintained high stability. Furthermore, the sensor successfully detected CGA in human fluids (blood) and food samples (coffee beans, carrots, and tea) with satisfactory recoveries. Its integration with screen-printed carbon electrodes (SPCE) highlights its potential for real-time, portable sensing applications in biological and environmental analysis.
溶剂辅助氧化铁纳米颗粒选择性电化学检测绿原酸的实际样品
氧化铁(α-Fe2O3,赤铁矿)是一种广泛应用、经济高效、高稳定性的p型半导体,具有广泛的应用潜力。由于其优异的电化学性能,赤铁矿在有毒污染物的检测中得到了广泛的应用。然而,优化其合成条件可以提高其电催化效率,使其成为一种很有前途的电化学传感材料。方法采用溶剂热法制备了赤铁矿(α-Fe₂O₃)纳米颗粒。采用PXRD, FT-IR, BET, XPS, FE-SEM和HR-TEM等技术对其结构和形态进行了表征。利用循环伏安法(CV)和方波伏安法(SWV)评价了电化学性能,并利用电化学阻抗谱(EIS)分析了电子传递动力学。在优化条件下,对Fe₂O₃-E(以乙醇为溶剂制备的氧化铁)修饰的GCE(玻碳电极)传感器进行了绿原酸(CGA)检测试验。所研制的Fe₂O₃-E修饰的GCE传感器具有良好的CGA检测电催化活性,检测限低(0.0066µM),线性范围宽(0.05 ~ 668µM)。尽管存在潜在的干扰,它仍具有良好的选择性和较高的稳定性。此外,该传感器还成功地检测了人体液体(血液)和食物样本(咖啡豆、胡萝卜和茶)中的CGA,回收率令人满意。它与丝网印刷碳电极(SPCE)的集成突出了其在生物和环境分析中的实时、便携式传感应用的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


