Reprogramming immunity: Emerging therapeutic frontiers and clinical trial advances of CAR-T cell therapy in autoimmune diseases
引用次数: 0
Abstract
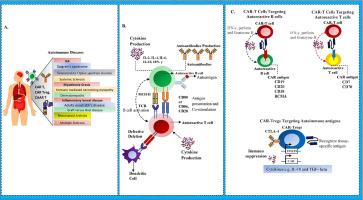
Autoimmune diseases result from a breakdown in immune tolerance, leading to chronic activation of autoreactive lymphocytes. Conventional therapies offer symptomatic relief but rarely achieve durable remission. Chimeric antigen receptor (CAR)-T cell therapy, originally developed for hematologic malignancies, is now being repurposed to selectively deplete pathogenic immune subsets in autoimmunity. This review explores how CAR-T cells may not only eliminate autoreactive B and T cells but also reprogram immune homeostasis toward long-term tolerance. Recent clinical success with CD19-directed CAR-T cells in refractory systemic lupus erythematosus underscores this paradigm shift. Furthermore, next-generation constructs including dual CARs, inhibitory CARs (iCARs), and CAR-Tregs offer innovative strategies to balance cytotoxicity with regulatory control. Key challenges such as antigen escape, off-target effects, and therapeutic accessibility are critically analyzed within current translational and clinical landscapes. This work advocates for a mechanistically guided redefinition of autoimmune therapy through precision-engineered immune reprogramming.
免疫重编程:CAR-T细胞治疗自身免疫性疾病的新治疗前沿和临床试验进展
自身免疫性疾病源于免疫耐受的破坏,导致自身反应性淋巴细胞的慢性激活。传统疗法能缓解症状,但很少能持久缓解。嵌合抗原受体(CAR)-T细胞疗法最初用于血液系统恶性肿瘤,现在被重新用于选择性地消耗自身免疫中的致病性免疫亚群。这篇综述探讨了CAR-T细胞不仅可以消除自身反应性B细胞和T细胞,还可以重新编程免疫稳态以实现长期耐受性。最近cd19靶向CAR-T细胞治疗难治性系统性红斑狼疮的临床成功强调了这种范式转变。此外,下一代构建物包括双car、抑制性car (iCARs)和CAR-Tregs,提供了平衡细胞毒性和调节控制的创新策略。关键的挑战,如抗原逃逸,脱靶效应,治疗可及性在当前的翻译和临床景观进行了批判性分析。这项工作提倡通过精确工程免疫重编程对自身免疫治疗进行机械指导的重新定义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


