Progress towards a prodrug/enzyme intranasal delivery system for rapid prevention/reversal of seizure emergency
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
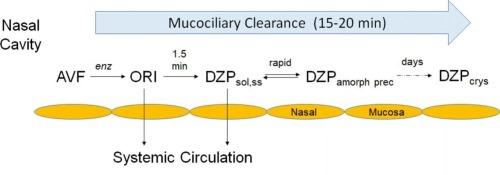
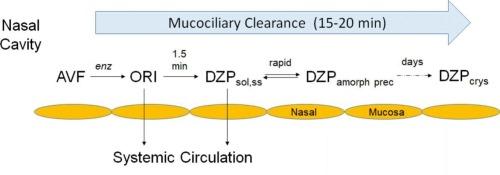
Sporadic epileptic seizure emergencies (SEs) require rapid treatment to prevent the emergence of status epilepticus, a condition that can present morbidities and possible mortality. First-line treatment of SEs has involved IV administration of benzodiazepines (BZDs) to the patient in the emergency department of a hospital, outpatient buccal administration, or administration of an outpatient rectal gel. These therapies are suboptimal for a variety of reasons, and recently, BZD nasal sprays have been introduced. Such sprays must solve the problem of poor aqueous solubility of BZDs. The present sprays contain organic excipients, which can be irritating, and the BZDs show either moderate bioavailability or slow absorption. In this Perspective we review work on a purely aqueous formulation containing avizafone (AVF), a prodrug of the BZD diazepam (DZP), and the converting enzyme human amino peptidase B (APB). Mixing these two ingredients at the point of administration creates a supersaturated aqueous DZP solution, which is rapidly absorbed across the nasal mucosa without the irritation mentioned above. We review characterization of the enzyme reaction and in vivo studies in which Sprague-Dawley rats were administered the AVF/APB formulation intranasally. Tmax values of DZP in both plasma and brain were both about 8 min, and brain concentrations appear to have reached 70 ng/g, which is considered adequate for controlling seizures within 2 min. We also describe a method to ensure the long-term shelf stability of the ingredients. These results encourage further development of the AVF/APB formulation. Studies with APB and an alternative BZD, midazolam (MDZ), were not as promising due to a kinetic bottleneck following the enzyme reaction.
用于快速预防/逆转癫痫紧急情况的前药/酶鼻内给药的进展
散发性癫痫发作紧急情况(SEs)需要快速治疗,以防止癫痫持续状态的出现,这种情况可能出现发病率和可能的死亡率。SEs的一线治疗包括在医院急诊科给病人静脉注射苯二氮卓类药物(BDZs)、门诊口腔给药或门诊直肠凝胶给药。由于各种原因,这些疗法都不是最佳的,最近,BZD鼻喷雾剂已经被引入。这种喷雾剂必须解决BZDs水溶性差的问题。目前的喷雾剂含有有机赋形剂,这可能是刺激性的,并且BZDs显示出中等的生物利用度或缓慢的吸收。在这方面,我们回顾了纯水制剂的工作,其中含有avizafone (AVF), BZD地西泮(DZP)的前药,和转化酶人氨基肽酶B (APB)。在给药点混合这两种成分会产生过饱和的DZP水溶液,它会迅速被鼻黏膜吸收,而不会产生上述刺激。我们回顾了酶反应的特征和Sprague-Dawley大鼠鼻内给药AVF/APB制剂的体内研究。DZP在血浆和脑中的Tmax值均约为8 min,脑浓度似乎已达到70 ng/kg,这被认为足以在2 min内控制癫痫发作。我们还描述了一种确保成分长期货架稳定性的方法。这些结果鼓励了AVF/APB制剂的进一步发展。由于酶反应后的动力学瓶颈,用APB和另一种BZD咪达唑仑(MDZ)进行的研究并不那么有希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


