Pharmacokinetics and molecular-level insights into 5-Methyl-3-(trifluoromethyl)-1H-pyrazole for anticancer action: Spectroscopic profiling, solvent interactions, topological analysis and ADME–QSAR predictions
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2025-09-12
DOI:10.1016/j.saa.2025.126940
引用次数: 0
Abstract
This study investigated the structural and spectroscopic characteristics of 5-methyl-3-(trifluoromethyl)-1H-pyrazole through PXRD/Rietveld refinement and DFT methods. A PES scan revealed two conformers, with Conformer I exhibiting the lowest energy at −602.619 Hartree (a.u.). Structural and topological analyses, including AIM, LOL, ELF, and RDG, were conducted, extended to dimeric and trimeric forms to elucidate intermolecular interactions. Vibrational analysis (FT-IR and FT-Raman) was performed to identify functional groups and bonding features, while 1H/13C NMR studies were conducted to analyse chemical shift variations associated with the dimeric and trimeric forms. Solvent effect on reactivity was assessed using FMO analysis. Intermolecular hydrogen bonding and crystal structure were characterized using Hirshfeld-surface analysis. Experimental and theoretical UV–Vis absorption spectra across various solvents have pinpointed regions of electronic transitions. Solute-solvent interaction parameters ( and ) with a correlation (R2 = 0.93789) revealed a strong influence of solvent polarity and hydrogen-bonding properties on the absorption maxima (λmax) of the compound. ADMET predictions indicate strong drug-development potential with high blood–brain barrier (BBB) penetration, low metabolic liability, and good oral bioavailability, but minimal hERG inhibition. The compound showed binding energies of −8.47 and − 8.01 kcal mol−1 against MAP3K14 (NIK) target proteins, suggesting potential anticancer activity.
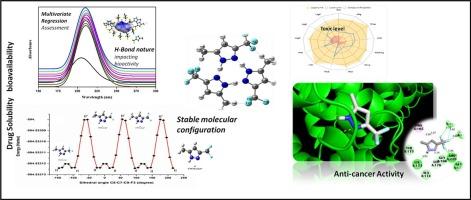
5-甲基-3-(三氟甲基)- 1h -吡唑抗癌作用的药代动力学和分子水平:光谱分析、溶剂相互作用、拓扑分析和ADME-QSAR预测。
本研究通过PXRD/Rietveld细化和DFT方法研究了5-甲基-3-(三氟甲基)- 1h -吡唑的结构和光谱特性。PES扫描显示了两个构象,其中构象I的能量最低,为-602.619 Hartree (a.u)。我们进行了结构和拓扑分析,包括AIM、LOL、ELF和RDG,并扩展到二聚体和三聚体形式,以阐明分子间的相互作用。振动分析(FT-IR和FT-Raman)用于识别官能团和键合特征,而1H/13C NMR研究用于分析与二聚体和三聚体形式相关的化学位移变化。用FMO分析评价溶剂对反应性的影响。分子间氢键和晶体结构用赫希菲尔德表面分析表征。实验和理论紫外-可见吸收光谱跨越各种溶剂已经确定了区域的电子跃迁。溶质-溶剂相互作用参数(π∗,α和β)呈相关关系(R2 = 0.93789),表明溶剂极性和氢键性质对化合物的吸收最大值(λmax)有很大影响。ADMET预测表明,具有高血脑屏障(BBB)渗透、低代谢负荷和良好的口服生物利用度,但hERG抑制最小的药物开发潜力。该化合物对MAP3K14 (NIK)靶蛋白的结合能分别为-8.47和- 8.01 kcal mol-1,具有潜在的抗癌活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


