Molecular mechanism underlying alcohol's residual effects: The role of acetaldehyde in mitochondrial dysfunction at synapses in mouse brain cortex
IF 2.9
4区 医学
Q3 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
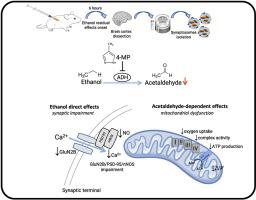
Alcohol residual effects impose significant physiological and cognitive burdens due to acute ethanol exposure; however, its underlying mechanisms remain poorly understood. This study investigates the role of acetaldehyde, the main ethanol metabolite, in driving mitochondrial dysfunction and synaptic impairment during hangover onset. Using a mice model, we evaluated the effects of ethanol (3.8 g/kg) and the alcohol dehydrogenase inhibitor 4-methylpyrazole (4-MP) on brain cortex synaptosomes.
Ethanol exposure significantly elevated serum acetaldehyde compared with control (p < 0.05), and induced mitochondrial dysfunction, as evidenced by impaired respiration (30 % decrease in basal O2 uptake vs. control), mitochondrial membrane depolarization and reduced ATP production (50 % decrease vs. control). These effects were mitigated by pre-treatment with 4-MP, which normalized acetaldehyde levels and partially restored mitochondrial function. Notably, ethanol downregulated synaptic proteins (nNOS, GluN2B, PSD-95; p < 0.05), but 4-MP failed to prevent this reduction, suggesting that acetaldehyde would not be involved in synaptic proteins alterations. Further, ethanol disrupted calcium homeostasis and nitric oxide (NO) content. Interestingly, 4-MP alone also reduced calcium uptake and NO content (p < 0.05), indicating potential off-target effects on neuronal signaling.
While the reduction in acetaldehyde levels preserved mitochondrial integrity, its inability to rescue synaptic protein loss highlights the complexity of hangover pathology, involving both acetaldehyde-dependent and -independent mechanisms. Our findings underscore acetaldehyde's pivotal role in hangover-associated mitochondrial dysfunction but reveal divergent pathways in synaptic impairment. These insights advance the search for targeted hangover therapies by delineating acetaldehyde-dependent toxicity.
酒精残留效应的分子机制:乙醛在小鼠脑皮质突触线粒体功能障碍中的作用
由于急性乙醇暴露,酒精残留效应造成显著的生理和认知负担;然而,其潜在机制仍然知之甚少。本研究探讨了主要的乙醇代谢物乙醛在宿醉发作时驱动线粒体功能障碍和突触损伤中的作用。通过小鼠模型,我们评估了乙醇(3.8 g/kg)和乙醇脱氢酶抑制剂4-甲基吡唑(4-MP)对大脑皮层突触体的影响。与对照组相比,乙醇暴露显著升高血清乙醛(p2摄取与对照组相比),线粒体膜去极化和ATP生成减少(与对照组相比减少50%)。4-MP预处理可以减轻这些影响,使乙醛水平正常化,并部分恢复线粒体功能。值得注意的是,乙醇下调突触蛋白(nNOS, GluN2B, PSD-95; p
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Alcohol
医学-毒理学
CiteScore
4.60
自引率
4.30%
发文量
74
审稿时长
15.6 weeks
期刊介绍:
Alcohol is an international, peer-reviewed journal that is devoted to publishing multi-disciplinary biomedical research on all aspects of the actions or effects of alcohol on the nervous system or on other organ systems. Emphasis is given to studies into the causes and consequences of alcohol abuse and alcoholism, and biomedical aspects of diagnosis, etiology, treatment or prevention of alcohol-related health effects.
Intended for both research scientists and practicing clinicians, the journal publishes original research on the neurobiological, neurobehavioral, and pathophysiological processes associated with alcohol drinking, alcohol abuse, alcohol-seeking behavior, tolerance, dependence, withdrawal, protracted abstinence, and relapse. In addition, the journal reports studies on the effects alcohol on brain mechanisms of neuroplasticity over the life span, biological factors associated with adolescent alcohol abuse, pharmacotherapeutic strategies in the treatment of alcoholism, biological and biochemical markers of alcohol abuse and alcoholism, pathological effects of uncontrolled drinking, biomedical and molecular factors in the effects on liver, immune system, and other organ systems, and biomedical aspects of fetal alcohol spectrum disorder including mechanisms of damage, diagnosis and early detection, treatment, and prevention. Articles are published from all levels of biomedical inquiry, including the following: molecular and cellular studies of alcohol''s actions in vitro and in vivo; animal model studies of genetic, pharmacological, behavioral, developmental or pathophysiological aspects of alcohol; human studies of genetic, behavioral, cognitive, neuroimaging, or pathological aspects of alcohol drinking; clinical studies of diagnosis (including dual diagnosis), treatment, prevention, and epidemiology. The journal will publish 9 issues per year; the accepted abbreviation for Alcohol for bibliographic citation is Alcohol.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


