Synergistic inhibition of hepatocarcinogenesis by green alga Ulva lactuca polysaccharide and 5-fluorouracil targeted SERPINH1
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
The serpin family H member 1 (SERPINH1) as a collagen-specific molecular chaperone, plays a crucial role in the biosynthesis of collagen. However, its function in hepatocellular carcinoma (HCC) is largely unexplored.
Purpose
To elucidate the mechanism which the combination of Ulva lactuca polysaccharide (ULP) and 5-fluorouracil (5-FU) synergistically inhibits tumors via targeting SERPINH1.
Methods
This study employed in vitro (RAW264.7 and HepG2 cells) and in vivo (H22 tumor-bearing mouse and xenograft zebrafish) models to investigate the mechanisms behind the synergistic antitumor effects and attenuated cytotoxicity of the ULP and 5-FU combination. RNA sequencing (RNA-seq) coupled with bioinformatic analyses was employed to explore the potential carcinogenesis and tumor-suppressive roles of SERPINH1. Furthermore, siRNA-mediated knockdown of SERPINH1 was performed to confirm its functional significance in HCC.
Results
A combination of ULP and 5-FU augments tumor cell inhibition and alleviates oxidative stress damage caused by chemotherapy. ULP and 5-FU inhibited collagen secretion by downregulating SERPINH1 expression, thereby impairing extracellular matrix (ECM) deposition. Consequently, this led to the suppression of invasion and migration in HepG2 cells.
Conclusion
ULP is identified as a novel natural agent that synergizes with 5-FU to suppress tumor progression, primarily by modulating the ECM. The combination treatment targets SERPINH1, inhibiting collagen-mediated ECM deposition and consequently reducing tumor cell migration and invasion.
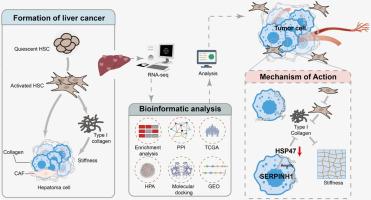
绿藻多糖与5-氟尿嘧啶靶向SERPINH1对肝癌发生的协同抑制作用。
背景:serpin家族H成员1 (SERPINH1)作为一种胶原特异性分子伴侣,在胶原的生物合成中起着至关重要的作用。然而,其在肝细胞癌(HCC)中的功能在很大程度上是未知的。目的:阐明ULP与5-氟尿嘧啶(5-FU)联合靶向SERPINH1协同抑制肿瘤的作用机制。方法:采用体外(RAW264.7和HepG2细胞)和体内(H22荷瘤小鼠和异种移植斑马鱼)模型,探讨ULP和5-FU联合抗肿瘤和降低细胞毒性的机制。采用RNA测序(RNA-seq)结合生物信息学分析来探索SERPINH1潜在的致癌和肿瘤抑制作用。此外,我们通过sirna介导的SERPINH1基因敲低来证实其在HCC中的功能意义。结果:ULP联合5-FU可增强肿瘤细胞抑制作用,减轻化疗引起的氧化应激损伤。ULP和5-FU通过下调SERPINH1表达抑制胶原分泌,从而损害细胞外基质(ECM)沉积。因此,这导致HepG2细胞的侵袭和迁移受到抑制。结论:ULP是一种新型的天然药物,可与5-FU协同抑制肿瘤进展,主要通过调节ECM。联合治疗以SERPINH1为靶点,抑制胶原介导的ECM沉积,从而减少肿瘤细胞的迁移和侵袭。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


