Low synaptic and neurosecretory proteins in cerebrospinal fluid in early parkinsonian disease
IF 3.2
3区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Background
The early pathogenesis of Parkinson's disease (PD) and the atypical parkinsonian disorders multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) is poorly understood, but presynaptic and axonal dysfunction are hypothesized to play a prominent role.
Objective
To identify synapse- and/or axonal dysfunction as indicated by cerebrospinal fluid (CSF) biomarker profiles and their clinical correlates in early-stage PD, MSA, and PSP.
Methods
Liquid chromatography mass spectrometry and enzyme-linked immunosorbent assay analyses of CSF samples from patients with early-stage PD (n = 38), MSA (n = 21), or PSP (n = 19), and age-matched, neurologically healthy controls (n = 30).
Results
Compared to controls, patients with early parkinsonian disorders had significantly reduced CSF levels of the synapse-associated proteins neuronal pentraxin-1 (NPTX1), amyloid precursor protein, and β-amyloid 42 (Aβ42), as well as the neurosecretory granin-derived proteins secretogranin-II, chromogranin-A, and secretogranin-VII. Among these, synapse-associated proteins correlated with non-motor features, while none correlated with age. CSF levels of the predominantly axonal proteins neurofilament light (NfL) and tau were elevated in patients with MSA or PSP. Reduced NPTX1 and Aβ42 distinguished PD from PSP, while elevated NfL and tau distinguished PSP and/or MSA from PD.
Conclusions
Low CSF levels of biomarkers associated with synaptic and neurosecretory function (e.g., NPTX1) implicate age-independent synaptic dysfunction as a shared, early feature in the pathogenesis of PD, MSA, and PSP. Such biomarkers may be particularly sensitive correlates of early non-motor dysfunction. Early axonal dysfunction is more pronounced in PSP and MSA than in PD.
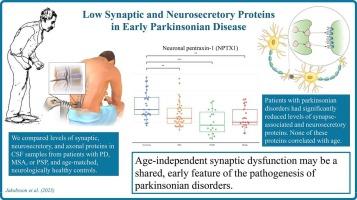
早期帕金森病脑脊液中突触和神经分泌蛋白含量低。
背景:帕金森病(PD)和非典型帕金森病多系统萎缩(MSA)和进行性核上性麻痹(PSP)的早期发病机制尚不清楚,但突触前和轴突功能障碍被假设在其中起着重要作用。目的:通过脑脊液(CSF)生物标志物及其临床相关性,确定早期PD、MSA和PSP患者的突触和/或轴突功能障碍。方法:液相色谱质谱法和酶联免疫吸附法分析了来自早期PD (n = 38)、MSA (n = 21)或PSP (n = 19)和年龄匹配的神经健康对照(n = 30)患者的脑脊液样本。结果:与对照组相比,早期帕金森病患者脑脊液中突触相关蛋白神经元戊素-1 (NPTX1)、淀粉样蛋白前体蛋白和β-淀粉样蛋白42 (Aβ42)以及神经分泌性颗粒蛋白衍生蛋白分泌颗粒蛋白ii、嗜铬颗粒蛋白a和分泌颗粒蛋白vii的水平显著降低。其中,突触相关蛋白与非运动特征相关,而与年龄无关。MSA或PSP患者脑脊液中主要轴突蛋白神经丝光(neurofilament light, NfL)和tau水平升高。降低的NPTX1和a - β42将PD与PSP区分开来,而升高的NfL和tau将PSP和/或MSA与PD区分开来。结论:脑脊液中与突触和神经分泌功能相关的生物标志物(如NPTX1)水平低,暗示年龄无关的突触功能障碍是PD、MSA和PSP发病机制的一个共同的早期特征。这些生物标志物可能与早期非运动功能障碍特别敏感。早期轴突功能障碍在PSP和MSA中比在PD中更为明显。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of the Neurological Sciences
医学-临床神经学
CiteScore
7.60
自引率
2.30%
发文量
313
审稿时长
22 days
期刊介绍:
The Journal of the Neurological Sciences provides a medium for the prompt publication of original articles in neurology and neuroscience from around the world. JNS places special emphasis on articles that: 1) provide guidance to clinicians around the world (Best Practices, Global Neurology); 2) report cutting-edge science related to neurology (Basic and Translational Sciences); 3) educate readers about relevant and practical clinical outcomes in neurology (Outcomes Research); and 4) summarize or editorialize the current state of the literature (Reviews, Commentaries, and Editorials).
JNS accepts most types of manuscripts for consideration including original research papers, short communications, reviews, book reviews, letters to the Editor, opinions and editorials. Topics considered will be from neurology-related fields that are of interest to practicing physicians around the world. Examples include neuromuscular diseases, demyelination, atrophies, dementia, neoplasms, infections, epilepsies, disturbances of consciousness, stroke and cerebral circulation, growth and development, plasticity and intermediary metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


