Application of integrated in vitro fecal cultivation and 16S rRNA gene sequencing for optimizing beneficial gut microbiota combinations
IF 1.9
4区 生物学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Obesity is a global health concern closely linked to changes in gut microbiota and other metabolic disorders. In this study, we used 16S rRNA gene amplicon sequencing to analyze the gut microbiota composition of normal and obese fecal samples. We further investigated the influence of a beneficial gut microbiota combination, consisting of Phocaeicola vulgatus KBL981, Roseburia intestinalis KBL982, and Akkermansia muciniphila KBL983, strains considered beneficial for obesity management, using in vitro feces cultivation. Results showed a significant difference between the microbiota composition of normal and obesity fecal samples, with the obesity group displaying lower diversity and higher levels of pathogenic Shigella boydii and inflammation-related Bacteroides fragilis. The ideal beneficial gut microbiota mixture (1:1:100) significantly enhanced microbial diversity, balanced the short-chain fatty acid ratio, promoted the growth of beneficial bacteria, and inhibited the growth of harmful obesity-related species. However, oversupplementation of beneficial microbiota reduced the microbial diversity and disrupted the microbial ecosystem. The current study demonstrates that a combined beneficial gut microbiota treatment can help manage obesity-related dysbiosis.
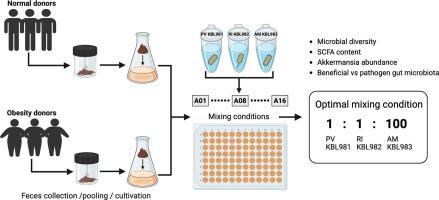
应用体外粪便综合培养和16S rRNA基因测序优化肠道有益菌群组合。
肥胖是一个全球性的健康问题,与肠道微生物群的变化和其他代谢紊乱密切相关。在本研究中,我们使用16S rRNA基因扩增子测序分析了正常和肥胖粪便样本的肠道微生物群组成。我们进一步研究了有益肠道菌群组合的影响,包括Phocaeicola vulgatus KBL981、Roseburia肠子KBL982和Akkermansia muciniphila KBL983,这些菌株被认为对肥胖管理有益。结果显示,正常和肥胖粪便样本的微生物群组成存在显著差异,肥胖组的微生物群多样性较低,致病性博氏志贺氏菌和炎症相关的脆弱拟杆菌含量较高。理想的肠道有益菌群混合物(1:1:100)显著增强了微生物多样性,平衡了短链脂肪酸比例,促进了有益菌的生长,抑制了肥胖相关有害菌的生长。然而,过量补充有益菌群会降低微生物多样性,破坏微生物生态系统。目前的研究表明,联合有益的肠道微生物群治疗可以帮助控制肥胖相关的生态失调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of microbiological methods
生物-生化研究方法
CiteScore
4.30
自引率
4.50%
发文量
151
审稿时长
29 days
期刊介绍:
The Journal of Microbiological Methods publishes scholarly and original articles, notes and review articles. These articles must include novel and/or state-of-the-art methods, or significant improvements to existing methods. Novel and innovative applications of current methods that are validated and useful will also be published. JMM strives for scholarship, innovation and excellence. This demands scientific rigour, the best available methods and technologies, correctly replicated experiments/tests, the inclusion of proper controls, calibrations, and the correct statistical analysis. The presentation of the data must support the interpretation of the method/approach.
All aspects of microbiology are covered, except virology. These include agricultural microbiology, applied and environmental microbiology, bioassays, bioinformatics, biotechnology, biochemical microbiology, clinical microbiology, diagnostics, food monitoring and quality control microbiology, microbial genetics and genomics, geomicrobiology, microbiome methods regardless of habitat, high through-put sequencing methods and analysis, microbial pathogenesis and host responses, metabolomics, metagenomics, metaproteomics, microbial ecology and diversity, microbial physiology, microbial ultra-structure, microscopic and imaging methods, molecular microbiology, mycology, novel mathematical microbiology and modelling, parasitology, plant-microbe interactions, protein markers/profiles, proteomics, pyrosequencing, public health microbiology, radioisotopes applied to microbiology, robotics applied to microbiological methods,rumen microbiology, microbiological methods for space missions and extreme environments, sampling methods and samplers, soil and sediment microbiology, transcriptomics, veterinary microbiology, sero-diagnostics and typing/identification.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


