Targeting the blood-brain barrier with lipoprotein-mimicking nanoparticles loaded with flurbiprofenaxetil and coated with apolipoprotein E3
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
In previous studies, it has been demonstrated that lipoprotein-mimicking nanoparticles with a solid lipid core of cholesteryl oleate, a lecithin coating and adsorptively bound apolipoprotein E3 (ApoE) may serve as a potential vehicle for drug delivery to the central nervous system. In this study, the impact of drug characteristics, particularly lipophilicity, was evaluated to achieve a stable incorporation of model drugs into these lipid-based nanoparticles (LNPs). This study explored the lipophilicity of flurbiprofen, a potential drug in the treatment of Alzheimer’s disease (AD), and its prodrug flurbiprofenaxetil across varying pH levels. Our findings highlight how flurbiprofen’s lipophilicity was influenced by its protonation state, affecting its incorporation into LNPs and consequently its release behaviour under physiological conditions, while flurbiprofenaxetil showed minimal variations due to its chemical structure. We also investigated the interaction between lipoprotein mimicking nanoparticles and primary porcine brain capillary endothelial cells to improve drug delivery across the blood-brain barrier (BBB). Permeation studies indicated that modification with ApoE enhanced the bidirectional permeability of LNPs across the BBB through receptor-mediated transcytosis. Furthermore, we demonstrated and identified the uptake mechanism involving the low density lipoprotein receptor-related protein 1 (LRP1), allowing these LNPs to be recognized by the same receptors as endogenous lipoproteins. Overall, these findings highlight the potential of ApoE modified LNPs as a promising strategy for targeted drug delivery to the brain.
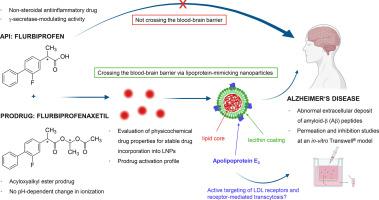
载氟比洛芬酯并包被载脂蛋白E3的模拟脂蛋白纳米颗粒靶向血脑屏障。
在先前的研究中,已经证明具有油酸胆固醇固体脂质内核、卵磷脂涂层和吸附结合的载脂蛋白E3 (ApoE)的脂蛋白模拟纳米颗粒可能作为药物递送到中枢神经系统的潜在载体。在这项研究中,评估了药物特性的影响,特别是亲脂性,以实现模型药物与这些脂基纳米颗粒(LNPs)的稳定结合。本研究探讨了氟比洛芬(一种治疗阿尔茨海默病(AD)的潜在药物)及其前药氟比洛芬酯在不同pH水平下的亲脂性。我们的研究结果强调了氟比洛芬的亲脂性如何受到其质子化状态的影响,影响其与LNPs的结合,从而影响其在生理条件下的释放行为,而氟比洛芬酯由于其化学结构而表现出最小的变化。我们还研究了脂蛋白模拟纳米颗粒与原代猪脑毛细血管内皮细胞之间的相互作用,以改善药物通过血脑屏障(BBB)的传递。渗透性研究表明,ApoE修饰可通过受体介导的胞吞作用增强LNPs穿过血脑屏障的双向渗透性。此外,我们证明并确定了涉及低密度脂蛋白受体相关蛋白1 (LRP1)的摄取机制,使这些LNPs能够被相同的受体识别为内源性脂蛋白。总的来说,这些发现强调了ApoE修饰LNPs作为一种有前途的靶向药物输送到大脑的策略的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


