Self-regenerable zeolite Beta(35) for efficient adsorption and photocatalytic degradation of PFOA under simulated solar irradiation
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
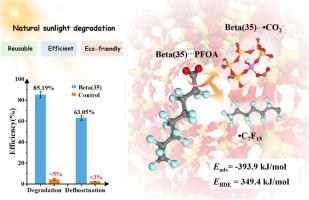
Perfluorooctanoic acid (PFOA), a priority emerging pollutant, necessitates efficient and green removal methods. While adsorption is cost-effective under mild conditions, the adsorbent treatment often causes additional costs and limits its applicability. This study employs a BEA-topology zeolite (Beta(35)) as a bifunctional adsorbent and photocatalyst for PFOA removal. Beta(35) at a dosage of 0.25 g/L achieved 90.7 % adsorption of 20 mg/L PFOA within 1 h, with adsorption capacities reaching 80 mg/g. Under natural sunlight irradiation, Beta(35) demonstrated 85.2 % degradation efficiency and 63.1 % defluorination efficiency for PFOA within 24 h. Additionally, the feasibility of treating trace PFOA-contaminated water was confirmed, where 2 g of Beta(35) effectively enriched and degraded 4 L of simulated groundwater containing 50 μg/L PFOA, achieving a degradation efficiency of 94.1 % within 48 h. Theoretical calculations and spectral analyses confirmed that PFOA adsorbs onto the acidic sites of Beta(35) through bidentate/bridging coordination, reducing the C![]() C bond dissociation energy at the carboxyl end to facilitate degradation. Besides, the complexation of Beta(35) and PFOA improved the photocatalytic performance of Beta(35), facilitating the formation of h+ and ROS (•OH and •O2−). Beta(35) remove aqueous PFOA via initial adsorption followed by photocatalytic degradation for self-regeneration, offering a sustainable and cost-effective solution for PFAS remediation.
C bond dissociation energy at the carboxyl end to facilitate degradation. Besides, the complexation of Beta(35) and PFOA improved the photocatalytic performance of Beta(35), facilitating the formation of h+ and ROS (•OH and •O2−). Beta(35) remove aqueous PFOA via initial adsorption followed by photocatalytic degradation for self-regeneration, offering a sustainable and cost-effective solution for PFAS remediation.
自再生沸石β(35)在模拟太阳照射下对PFOA的有效吸附和光催化降解
全氟辛酸(PFOA)是一种优先出现的污染物,必须采用高效和绿色的清除方法。虽然吸附在温和条件下具有成本效益,但吸附剂处理通常会导致额外的成本并限制其适用性。本研究采用bea拓扑分子筛(Beta(35))作为双功能吸附剂和光催化剂去除PFOA。β(35)在0.25 g/L的用量下,对20 mg/L PFOA的吸附在1 h内达到90.7%,吸附量达到80 mg/g。在自然日光照射下,β(35)在24 h内对PFOA的降解效率为85.2%,除氟效率为63.1%。此外,还证实了处理痕量PFOA污染水体的可行性,其中2 g β(35)可有效富集和降解4 L含50 μg/L PFOA的模拟地下水;理论计算和光谱分析证实,PFOA通过双齿/桥接配位吸附在β(35)的酸性位点上,降低羧基端的C-C键解离能,有利于降解。此外,β(35)与PFOA的络合作用提高了β(35)的光催化性能,促进了h+和ROS(•OH和•O2−)的形成。β(35)通过初始吸附和光催化降解来去除水中的PFOA,从而实现自再生,为PFAS的修复提供了可持续和经济的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


