Temporal Immunomodulatory Hydrogel Regulating the Immune-Osteogenic Cascade for Infected Bone Defects Regeneration
IF 26.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Early pathogen clearance and immunomodulation are critical for the restoration of infected bone defects. Conventional osteoimmunomodulatory strategies mainly emphasize M2 macrophage-mediated bone regeneration, neglecting the pivotal role of early-stage M1 macrophage-activated immune response in microbial elimination. This oversight ultimately compromises repair efficacy in infected bone defects. Herein, a temporal immunomodulatory hydrogel is developed to regulate the immune-osteogenic microenvironment for the repair of infected bone defects. The hydrogel is rapidly formed by crosslinking of acrylate-modified engineered protein with oxidized sodium alginate, mimicking extracellular matrix architecture to promote cell adhesion, angiogenesis, and osteogenesis. To achieve temporal ion release, zinc-based nanoparticles mineralized with hydroxyapatite are incorporated within the hydrogel matrix. The early-stage release of Ca2+ promotes M1 polarization to inhibit infection, while sustained release of Zn2+ induces M2 polarization to promote osteogenic differentiation. This system further exhibits antioxidant and antibacterial properties, ensuring comprehensive immunomodulation across the bone healing process. In a rat model of infected cranial defects, the hydrogel effectively remodels the osteoimmune microenvironment, suppresses infection, and facilitates vascularized bone regeneration. This work highlights a temporal immunomodulatory strategy for infected bone repair and offers new insights into the design of advanced osteoimmunomodulatory biomaterials.
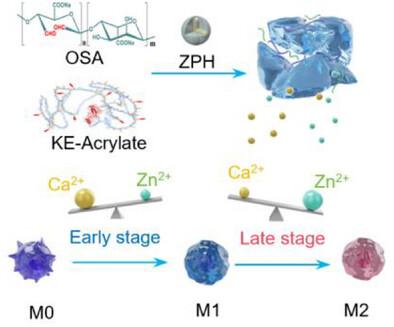
时间免疫调节水凝胶调节感染骨缺损再生的免疫-成骨级联
早期病原体清除和免疫调节对感染骨缺损的修复至关重要。传统的骨免疫调节策略主要强调M2巨噬细胞介导的骨再生,而忽视了早期M1巨噬细胞激活的免疫应答在微生物消除中的关键作用。这种疏忽最终会损害受感染骨缺损的修复效果。本研究开发了一种时间免疫调节水凝胶,用于调节免疫成骨微环境,修复受感染的骨缺损。水凝胶是通过丙烯酸酯修饰的工程蛋白与氧化海藻酸钠交联快速形成的,模拟细胞外基质结构,促进细胞粘附、血管生成和成骨。为了实现时间离子释放,锌基纳米颗粒与羟基磷灰石矿化被纳入水凝胶基质。Ca2+的早期释放促进M1极化抑制感染,而Zn2+的持续释放诱导M2极化促进成骨分化。该系统进一步表现出抗氧化和抗菌特性,确保在整个骨愈合过程中进行全面的免疫调节。在大鼠感染颅骨缺损模型中,水凝胶有效地重塑骨免疫微环境,抑制感染,促进血管化骨再生。这项工作强调了感染骨修复的时间免疫调节策略,并为高级骨免疫调节生物材料的设计提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


