Construction of meta-Disubstituted Triaryls via Iodine-Catalyzed Oxidative Aromatization Coupling of Cycloalkenes with Indoles
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
meta-Disubstituted triaryls are privileged scaffolds in bioactive molecules and functional materials, but their efficient synthesis remains challenging, due to limitations in regioselectivity and reliance on prefunctionalized substrates or transition metals. Herein, an iodine-catalyzed oxidative aromatization strategy is reported for the construction of meta-triaryls via direct coupling of readily available cycloalkenes with indoles. This protocol enables regioselective CC bond formation at the indole C3-position and a subsequent dehydrogenative desaturation of the cycloalkene component to form the meta-substituted arene motif. The method features broad substrate scope, accommodating diverse substituted cycloalkenes and indoles. Key advantages include economical catalyst (HI as iodine source), avoidance of precious metals, and operational simplicity. Additionally, the products can undergo an array of synthetic transformations, including heterocycle skeleton editing to quinolines, halogenations, and photoredox functionalizations, which highlight the potential applications of this strategy.
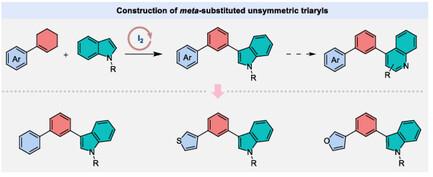
碘催化环烯烃与吲哚氧化芳构偶联构建间二取代三芳基
间二取代三芳基是生物活性分子和功能材料的首选支架,但由于区域选择性的限制和对预功能化底物或过渡金属的依赖,它们的高效合成仍然具有挑战性。本文报道了一种碘催化氧化芳构化策略,通过易得的环烯烃与吲哚的直接偶联来构建间三芳基。该方案使得在吲哚c3位置形成区域选择性C - _ - C键和随后的环烯烃组分脱氢脱饱和形成间取代芳烃基序。该方法具有广泛的底物范围,可容纳多种取代的环烯烃和吲哚。主要优点包括经济的催化剂(HI作为碘源),避免贵金属,操作简单。此外,这些产物可以经过一系列的合成转化,包括杂环骨架编辑到喹啉、卤化和光氧化还原功能化,这突出了该策略的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


