Bulk-surface engineering of PEO-based electrolytes via controlled [NMP-Li+] coordination for room-temperature quasi-solid-state lithium metal batteries
IF 14.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The practical deployment of poly(ethylene oxide) (PEO)-based solid electrolytes is significantly hampered by their low room-temperature ionic conductivity, stemming from high PEO crystallinity and strong ethylene oxide (EO)-Li⁺ coordination. Herein, we introduce a bulk-surface engineering strategy utilizing controlled N-methylpyrrolidone (NMP) coordination and a simple two-step drying process. This approach creates an asymmetric-structured PEO electrolyte where distinct molecular environments are engineered in the bulk and at the surface. Experimental and theoretical analyses reveal that controlled NMP coordination during the initial drying establishes a bulk [NMP-Li⁺] solvation structure that effectively weakens EO-Li⁺ binding, dramatically enhancing Li⁺ transport kinetics. Crucially, the subsequent drying phase intentionally depletes NMP from the electrolyte surface, forming a unique NMP-deficient phase. This engineered surface eliminates the thermodynamic instability of NMP towards Li metal, fostering a robust solid electrolyte interphase. Consequently, the asymmetric electrolyte (NP-x) achieves a high room-temperature ionic conductivity (0.14 mS cm−1) and Li⁺ transference number (0.41). Symmetrical Li-Li cells demonstrate ultra-stable cycling exceeding 2000 h at 25°C. The obtained solid-state Li-LiFePO4 cells deliver a high specific capacity (158.4 mAh g−1 at 0.2 C) with 89% capacity retention over 500 cycles, and maintain stable cyclability even at 0 and –15°C. This solvent coordination-mediated bulk-surface decoupling offers a fresh perspective for enhancing the Li+ transport and the interface stability of PEO-based electrolyte.
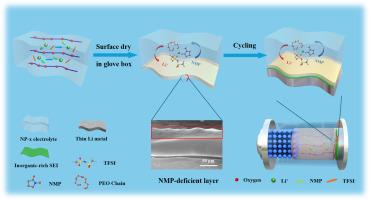
室温准固态锂金属电池用可控[NMP-Li+]配位peo基电解质的体面工程
聚环氧乙烷(PEO)基固体电解质的实际部署受到高PEO结晶度和强环氧乙烷(EO)-Li⁺配位性的低室温离子电导率的严重阻碍。在此,我们介绍了一种利用可控n -甲基吡咯烷酮(NMP)配位和简单的两步干燥过程的体表面工程策略。这种方法创造了一种不对称结构的PEO电解质,其中在主体和表面设计了不同的分子环境。实验和理论分析表明,在初始干燥过程中,可控的NMP配位建立了一种体状的[NMP-Li +]溶剂化结构,有效地减弱了EO-Li +的结合,极大地增强了Li +的传输动力学。至关重要的是,随后的干燥阶段有意地从电解质表面耗尽NMP,形成独特的NMP缺乏阶段。这种工程表面消除了NMP对Li金属的热力学不稳定性,形成了坚固的固体电解质界面。因此,不对称电解质(NP-x)实现了高的室温离子电导率(0.14 mS cm−1)和Li⁺的转移数(0.41)。对称锂电池在25°C下表现出超过2000小时的超稳定循环。获得的固态Li-LiFePO4电池具有较高的比容量(0.2℃时为158.4 mAh g - 1),在500次循环中保持89%的容量,即使在0和-15℃下也能保持稳定的可循环性。这种溶剂配位介导的体-表面解耦为增强peo基电解质的Li+输运和界面稳定性提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


