Mechanistic understanding of ultrafast anomalous lithium-ion transport in supramolecular porous crystals from fused macrocycle cages
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
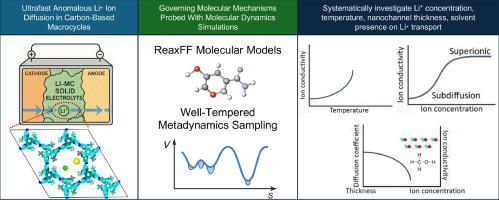
Fused macrocyclic cages have demonstrated excellent ion transport capabilities, yet the detailed mechanisms underlying their ion transport remain poorly understood, primarily due to the lack of experimental techniques capable of characterizing and visualizing processes at the molecular scale. In this study, we employ reactive molecular dynamics simulations to investigate the lithium-ion transport properties of a supramolecular porous crystal recently synthesized from fused macrocyclic cages arranged around a central prismatic core. Our simulations reveal that this crystal exhibits anomalous, non-Fickian subdiffusive ion transport behavior, which arises from the periodic trapping and release of Li⁺ ions at specific binding sites. Increasing the Li⁺ concentration initially enhances ionic conductivity, but conductivity declines once a critical concentration threshold is surpassed. Likewise, Li⁺ conductivity increases with temperature before declining at higher temperatures due to significant structural disturbances within the nanochannel. When methanol is introduced as a solvent, Li⁺ conductivity decreases because of ion clustering. Therefore, our findings indicate that the fused macrocyclic cage structure operates most effectively as a solid electrolyte in the absence of added solvents. Additionally, we observe that the diffusion coefficient diminishes as the thickness of the 1D nanochannel increases. These results provide important mechanistic insights for the design of all-solid-state lithium-ion batteries with ultrafast lithium transport characteristics.
熔融大环笼超分子多孔晶体中超快异常锂离子输运的机理研究
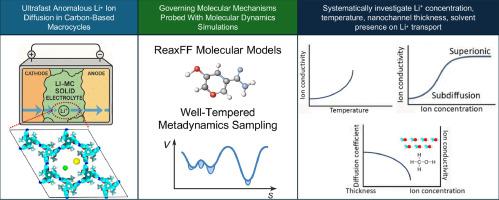
熔融大环笼已经证明了出色的离子传输能力,但其离子传输的详细机制仍然知之甚少,主要是由于缺乏能够在分子尺度上表征和可视化过程的实验技术。在这项研究中,我们采用反应分子动力学模拟来研究最近由围绕中心棱柱形核心排列的熔融大环笼合成的超分子多孔晶体的锂离子传输特性。我们的模拟表明,这种晶体表现出异常的、非菲克式的亚扩散离子传输行为,这是由Li +离子在特定结合位点的周期性捕获和释放引起的。增加Li +浓度最初可以增强离子电导率,但一旦超过临界浓度阈值,电导率就会下降。同样,Li⁺的电导率也会随着温度的升高而升高,然后由于纳米通道内的显著结构干扰而在更高温度下下降。当甲醇作为溶剂引入时,由于离子聚集,Li +的电导率降低。因此,我们的研究结果表明,在没有添加溶剂的情况下,熔融大环笼结构作为固体电解质运行最有效。此外,我们观察到扩散系数随一维纳米通道厚度的增加而减小。这些结果为设计具有超快锂输运特性的全固态锂离子电池提供了重要的机理见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


