Bioactive 2-arylbenzofuran and chalcone derivatives from Morus mesozygia Stapf
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The phytochemical investigation of the stem bark of Morus mesozygia afforded the fourteen known secondary metabolites moracin N (1), moracin S (2), mulberrofuran L (3), moracin C (4), moracin L (5), moracin M (6), moracin D (7), artopithecin A (8), isobavachalcone (9), morachalcone A (10), 2,2՛,4,4՛-tetrahydroxychalcone (11), 3β-acetoxy-urs-12-en-11-one (12), betulinic acid (13), and 4,4′-diphenylmethane-bis(methyl) carbamate (14). Their structures were elucidated by NMR spectroscopic and mass spectrometric analyzes and their cytotoxicity (CC₅₀ for HEKa, IMR-90, and HPrEC), anti-inflammatory (TNF-α, NF-κB, and NO inhibition), antibacterial (MIC), antitumor (IC₅₀), and antiviral (CPE-based and plaque reduction assays) activities were studied. Moracin D (7) showed potent anti-inflammatory activity towards the release of NF-κB (0.57 < IC50 < 1.21 μM). 3β-Acetoxy-urs-12-en-11-one (12) had potent antibacterial activity towards Bacillus subtilis and Micrococcus luteus, with MIC values of 12.71 and 15.59 μM, respectively. Moracin M (6) had the highest antitumor activity (IC50 = 19.80 μM) against SK-MEL-28 human melanoma cells. Isobavachalcone (9) exhibited activity against Human Rhinovirus 2 (HRV-2; IC50 = 7.01 μM) with a selectivity index (SI) = 9.1 as compared to HeLa cells. None of the compounds exhibited significant antiviral activity against respiratory syncytial virus (RSV) or herpes simplex virus type 2 (HSV-2). Out of the isolated compounds, moracin D (7), isobavachalcone (9) and marsformoxide B (12), may be considered to be promising leads for the development of anti-inflammatory (NF-κB), antiviral (HRV-2), and antibacterial (B. subtilis and M. luteus) agents, respectively.
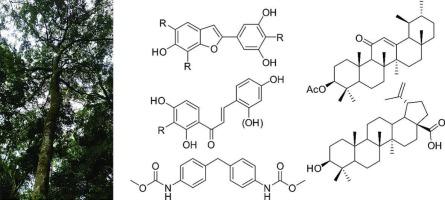
桑的生物活性2-芳基苯并呋喃和查尔酮衍生物。
对桑树茎皮的植物化学研究发现了14种已知的次生代谢产物moracin N(1)、moracin S(2)、mulberrofuran L(3)、moracin C(4)、moracin L(5)、moracin M(6)、moracin D(7)、artopithecin A(8)、isobavachalcone(9)、morachalcone A(10)、2,2 、4,4 -四羟基查尔酮(11)、3β-乙酰氧基-ur -12-en-11-one(12)、桦脂酸(13)和4,4′-二苯甲烷-双(甲基)氨基甲酸酯(14)。通过核磁共振波谱和质谱分析阐明了它们的结构,并研究了它们的细胞毒性(HEKa, IMR-90和HPrEC的CC₅0),抗炎(TNF-α, NF-κB和NO抑制),抗菌(MIC),抗肿瘤(IC₅0)和抗病毒(基于cpe和斑块减少试验)活性。Moracin D(7)对SK-MEL-28人黑色素瘤细胞释放NF-κB(0.57 50 50 = 19.80 μM)显示出强大的抗炎活性。Isobavachalcone(9)对人鼻病毒2型(HRV-2, IC50 = 7.01 μM)具有抑制作用,与HeLa细胞相比,其选择性指数(SI) = 9.1。这些化合物对呼吸道合胞病毒(RSV)或单纯疱疹病毒2型(HSV-2)均没有明显的抗病毒活性。在分离的化合物中,moracin D (7), isobavachalcone(9)和marsformoide B(12)可能被认为是开发抗炎(NF-κB),抗病毒(HRV-2)和抗菌(枯草芽孢杆菌和黄体芽孢杆菌)药物的有希望的线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


