Discovery of lipidated cysteine-based amphiphiles as bioinspired antimicrobial peptidomimetics against drug-resistant bacteria
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The emergence of multidrug-resistant bacteria poses a significant threat to modern medical practices and human health, creating an urgent demand for novel antibacterial agents. Inspired by host-defense peptides (HDPs), herein we employed fatty acid acylation to fine-tune the amphiphilic balance of cysteine-based amphiphilic compounds, synthesizing a series of antimicrobial peptidomimetics and evaluating their antimicrobial activity. Among them, 10c stood out due to its broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, as well as its low hemolytic activity. Like HDPs, it rapidly killed bacteria primarily via a membrane-disruptive action mode, with a low propensity to induce bacterial resistance. Additionally, its ease of synthesis, serum stability, and ability to address some of the challenges HDPs face in different physiological conditions may enhance its clinical applications. Importantly, 10c demonstrated significant therapeutic potential in mouse models of MRSA-induced pneumonia and keratitis. Overall, the present study provides new therapeutic strategies and identifies a potential lead compound for addressing antibiotic-resistant bacteria, offering novel insights for the development of next-generation antibacterial agents.
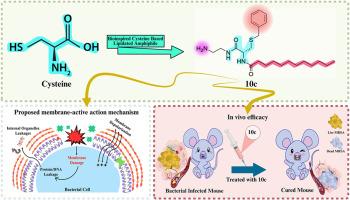
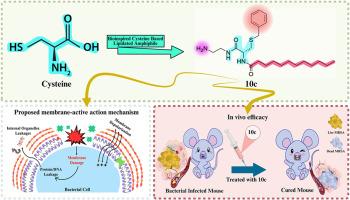
以脂化半胱氨酸为基础的两亲体作为抗耐药细菌的仿生抗菌肽的发现
耐多药细菌的出现对现代医疗实践和人类健康构成重大威胁,对新型抗菌剂产生了迫切需求。受宿主防御肽(宿主防御肽)的启发,我们利用脂肪酸酰化来微调半胱氨酸基两亲性化合物的两亲性平衡,合成了一系列抗菌类肽并评估了它们的抗菌活性。其中,10c因其对革兰氏阳性菌和革兰氏阴性菌均具有广谱抗菌活性,且溶血活性较低而脱颖而出。与HDPs一样,它主要通过膜破坏作用模式快速杀死细菌,诱导细菌耐药性的倾向较低。此外,其易于合成、血清稳定性和解决HDPs在不同生理条件下面临的一些挑战的能力可能会增强其临床应用。重要的是,10c在mrsa诱导的肺炎和角膜炎小鼠模型中显示出显著的治疗潜力。总的来说,本研究提供了新的治疗策略,并确定了一种潜在的先导化合物来解决抗生素耐药细菌,为下一代抗菌剂的开发提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


