An ionic liquid nanoemulsion transdermal delivery system for targeted melanoma therapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Melanoma is the deadliest form of skin malignancy. The existing transdermal treatment strategies are difficult to achieve effective concentration of drugs in deep-seated tumors, which seriously affects the effect of percutaneous treatment. The low efficacy of medications for treating melanoma and their percutaneous penetration into tumor tissues are the urgent problems to be solved in percutaneous treatment of skin malignancies. In this work, two dacarbazine/benzothiocycloheptane (DTIC/14) couplets were designed and synthesized, among which compound HIT-1 exhibited significantly cytotoxicity to B16-F10 (IC50 = 7.86 μM), and 10.3-fold stronger than DTIC. HIT-1 could significantly induce apoptosis and G0/G1 phase arrest of B16-F10 cells, showing a good anti-melanoma effect. Three self-assembled nanoemulsions (HIT-1/PM-MEs) were prepared on the basis of molecular simulation, among which HIT-1/PM-ME-1-1 had the best transdermal drug delivery effect. HIT-1/PM-ME-1-1 was not only 11.8-fold stronger than DTIC in terms of anti-B16-F10 activity, but also a good regulator of mRNA and protein expression of DNAJB1, HSPA1B, p53, Bcl-2 and Cleaved-caspase 3. Furthermore, the HIT-1/PM-ME-1-1 transdermal delivery system showed optimal antitumor effects and stimulated antitumor immune response. Altogether, these results strongly support that this transdermal drug delivery system provides a valuable new strategy for the treatment of cutaneous melanoma.
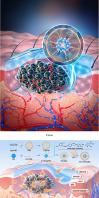
靶向黑色素瘤治疗的离子液体纳米乳透皮给药系统
黑色素瘤是最致命的皮肤恶性肿瘤。现有的经皮治疗策略难以在深部肿瘤中实现药物的有效浓度,严重影响了经皮治疗的效果。治疗黑色素瘤药物的低疗效及其经皮穿透肿瘤组织是皮肤恶性肿瘤经皮治疗中急需解决的问题。本文设计合成了两个达卡巴嗪/苯并噻吩环庚烷(DTIC/14)偶联,其中化合物HIT-1对B16-F10具有显著的细胞毒性(IC50 = 7.86 μM),比DTIC强10.3倍。HIT-1可显著诱导B16-F10细胞凋亡和G0/G1期阻滞,具有良好的抗黑色素瘤作用。在分子模拟的基础上制备了3种自组装纳米乳(HIT-1/PM-MEs),其中HIT-1/PM-ME-1-1的透皮给药效果最好。HIT-1/PM-ME-1-1不仅抗b16 - f10活性比DTIC强11.8倍,而且是DNAJB1、HSPA1B、p53、Bcl-2和Cleaved-caspase 3 mRNA和蛋白表达的良好调节剂。此外,HIT-1/PM-ME-1-1透皮给药系统表现出最佳的抗肿瘤效果,并刺激抗肿瘤免疫反应。总之,这些结果有力地支持这种经皮给药系统为治疗皮肤黑色素瘤提供了一种有价值的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


