Hierarchical ROS-scavenging platform breaks vicious cycle of stem cell senescence, angiogenesis arrest, and immune dysregulation in diabetic wounds
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Diabetic chronic wounds represent a formidable clinical challenge, driven by a pathological vicious cycle of reactive oxygen species (ROS)-induced oxidative stress, stem cell senescence, angiogenesis arrest, and immune dysregulation. Herein, we developed a hierarchical ROS-scavenging platform integrating nanoscale calcium hydride (CaH₂) within a microneedle (MN) patch to disrupt this degenerative cascade. Upon dissolution in wound exudate, CaH₂ nanoparticles react with water to generate sustained release of hydrogen gas (H₂) and calcium ions (Ca2+). The liberated H₂ directly neutralizes cytotoxic ROS, thereby reversing stem cell senescence and restoring their paracrine secretion of pro-angiogenic factors, while concomitantly reprogramming macrophages toward pro-regenerative M2 phenotypes. Simultaneously, Ca2+ synergizes with H₂ to activate endothelial cell migration and tubulogenesis, fostering robust vascular network formation. By concurrently resolving oxidative stress, stem cell senescence, angiogenesis arrest, and immune dysregulation, the CaH₂-MN system breaks the vicious cycle to reshape the wound microenvironment into a pro-regenerative state. In diabetic murine models, this approach accelerated wound closure, enhanced neovascularization, and reduced inflammatory infiltration. This multiscale intervention paradigm provides a blueprint for intercepting pathological cascades in diabetic wounds.
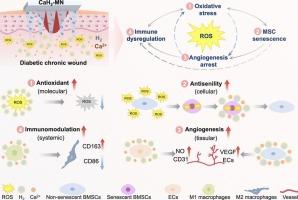
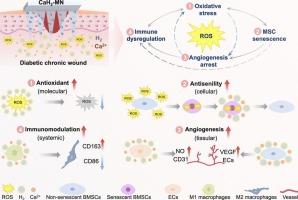
分层ros清除平台打破了糖尿病创面干细胞衰老、血管生成停滞和免疫失调的恶性循环
糖尿病慢性伤口是一个巨大的临床挑战,由活性氧(ROS)诱导的氧化应激、干细胞衰老、血管生成停止和免疫失调的病理恶性循环驱动。在此,我们开发了一个分层ros清除平台,将纳米级氢化钙(CaH₂)集成在微针(MN)贴片中,以破坏这种退化级联。在伤口渗出液中溶解后,CaH₂纳米颗粒与水反应,产生持续释放的氢气(H₂)和钙离子(Ca2+)。释放的H₂直接中和细胞毒性ROS,从而逆转干细胞衰老,恢复其旁分泌促血管生成因子,同时将巨噬细胞重编程为促再生的M2表型。同时,Ca2+与H₂协同作用,激活内皮细胞迁移和小管形成,促进强健的血管网络形成。通过同时解决氧化应激、干细胞衰老、血管生成停滞和免疫失调,CaH₂-MN系统打破了恶性循环,将伤口微环境重塑为促再生状态。在糖尿病小鼠模型中,这种方法加速了伤口愈合,增强了新生血管,减少了炎症浸润。这种多尺度干预模式为阻断糖尿病伤口的病理级联反应提供了蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


