Palladium-catalyzed carbonylative double cyclization leading to indole-fused ε- and ζ-lactones
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
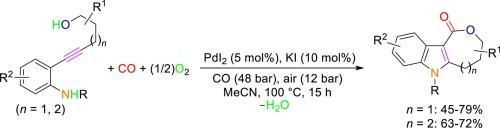
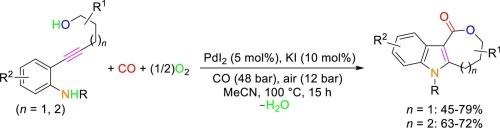
The reactivity of 5-(2-aminophenyl)-4-yn-1-ols and 6-(2-aminophenyl)-5-yn-1-ols under PdI2/KI-catalyzed oxidative carbonylation conditions has been studied. It has been found that, with 5 mol% of PdI2 and 0.5 equiv. of KI, under 60 bar of a 4:1 mixture of CO-air, in MeOH as the solvent at 100 °C for 24 h, a mixture of isomeric indole-fused ε-lactone and pyranoquinolinone derivatives was formed from 5-(2-aminophenyl)-4-yn-1-ols, deriving from divergent 5-endo-dig N-cyclization ‒ 7-O-cyclocarbonylation and 6-endo-dig O-cyclization – 6-N-cyclocarbonylation pathways, respectively. However, the process could be made selective toward the formation of the indole-fused ε-lactone (oxepinoindolone) product when performed in MeCN, thus establishing an unprecedented cyclization – seven-membered cyclocarbonylation process. Under the optimized conditions, a series of previously unknown 6-substituted 3,4,5,6-tetrahydro-1H-oxepino[4,3-b]indol-1-ones could be synthesized in moderate to satisfactory isolated yields (45–79 %) starting from differently substituted 5-(2-aminophenyl)-4-yn-1-ols. Under the same conditions, 6-(2-aminophenyl)-5-yn-1-ols were converted into indole-fused ζ-lactones through an even more challenging cyclization – eight-membered cyclocarbonylation process. The structures of three representative products have been confirmed by XRD analysis.
钯催化羰基双环化反应生成吲哚熔融ε-和ζ-内酯
研究了PdI2/ ki催化氧化羰基化条件下5-(2-氨基苯基)-4-氨基-1-醇和6-(2-氨基苯基)-5-氨基-1-醇的反应活性。结果表明,以5 摩尔%的PdI2和0.5等量的KI,在60 bar的4:1 CO-air混合物中,以甲醇为溶剂,在100 °C下反应24 h, 5-(2-氨基苯基)-4- n-1-醇分别从不同的5-内切n-环化- 7- o-环羰基化和6-内切o-环羰基化途径生成吲哚-融合ε-内酯和吡喹诺啉酮衍生物的混合物。然而,当在MeCN中进行时,该过程可以选择性地形成吲哚-融合的ε-内酯(oxepinoindolone)产物,从而建立了前所未有的环化-七元环羰基化过程。在优化的条件下,以不同取代的5-(2-氨基苯基)-4-氨基-1-醇为起始原料,可以合成一系列未知的6-取代的3,4,5,6-四氢- 1h -氧平苷[4,3-b]吲哚-1-酮,分离产率为45-79 %。在相同条件下,6-(2-氨基苯基)-5-炔-1-醇通过更具挑战性的环化-八元环羰基化过程转化为吲哚-融合的ζ-内酯。用XRD分析证实了三个代表性产物的结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


