FormulationMM: A universal computer-driven drug formulation platform
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Classical computer-aided drug design answers “Will this ligand bind?”, whereas computer-driven drug formulation answers the downstream but equally critical question “How can this drug be physically formulated and delivered?”, thereby bridging the long-standing gap between molecular modeling and drug formulation science through physics-based and fully automated multiscale simulation. To build this bridge, we introduce FormulationMM, an innovative platform utilizing real-world driven molecular modeling to explore drug formulation mechanisms. FormulationMM features a pharmaceutical formulation algorithm, an integrated excipient database, and robust modeling protocols, ensuring a streamlined workflow for the generation, simulation, and analysis of drug formulation. It automatically generates force field parameters for drug molecules and excipients, supporting six formulation types: cyclodextrin-drug inclusion, micelles, liposomes, solid dispersions, self-assembling drug nanoparticles, and transmembrane drug delivery systems. Our results closely match experimental findings and demonstrate high predictive accuracy and reliability. FormulationMM, accessible through a continuously updated website (https://formulationmm.computpharm.org), offers a practical platform to support drug formulation research and development, with the potential to advance the growing field of computer-driven drug formulation.
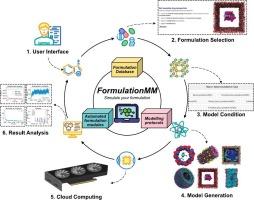
FormulationMM:一个通用的计算机驱动药物配方平台
经典的计算机辅助药物设计回答“这种配体会结合吗?”,而计算机驱动的药物配方则回答了下游但同样重要的问题:“这种药物如何在物理上制造和输送?”,从而通过基于物理的全自动多尺度模拟,弥合了分子建模和药物配方科学之间长期存在的差距。为了建立这个桥梁,我们推出了FormulationMM,一个利用现实世界驱动的分子模型来探索药物配方机制的创新平台。FormulationMM具有药物配方算法,全面的辅料数据库和强大的建模协议,确保药物配方生成,模拟和分析的简化工作流程。它自动生成药物分子和赋形剂的力场参数,支持六种剂型:环糊精-药物包合、胶束、脂质体、固体分散体、自组装药物纳米颗粒和跨膜给药系统。结果与实验结果吻合较好,具有较高的预测精度和可靠性。FormulationMM可通过不断更新的网站(https://formulationmm.computpharm.org)访问,它提供了一个实用的平台来支持药物配方的研究和开发,有可能推动计算机驱动的药物配方领域的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


