A Versatile Route to 3,3-Disubstituded Phthalides Via Arylogous Michael Addition Catalyzed by Organic Base
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The 3,3-disubstituted phthalide scaffold, a privileged structure in medicinal chemistry and natural product synthesis, serves as a key motif in many bioactive compounds. Leveraging the solvent-dependent pKa modulation of organic bases, this study introduces a mild, organocatalytic Michael addition protocol for coupling diverse 3-arylphthalides with Michael acceptors. The method demonstrates broad functional group tolerance, accommodating electron-donating and electron-withdrawing substituents. The versatility of this methodology is highlighted by mild reaction conditions, short reaction times, and gram-scale feasibility. Finally, the approach may open the gate to asymmetric catalysis, offering access to enantiomerically enriched phthalides.
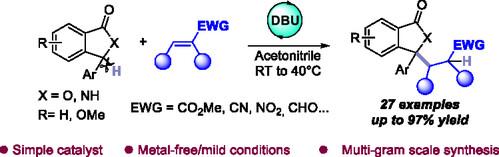
有机碱催化芳基迈克尔加成制备3,3-二取代邻苯二甲酸酯的通用途径
3,3-二取代邻苯酞支架是药物化学和天然产物合成中的一种特殊结构,是许多生物活性化合物的关键基序。利用有机碱的溶剂依赖性pKa调制,本研究引入了一种温和的有机催化Michael加成方案,用于将多种3-芳基苯酞与Michael受体偶联。该方法具有广泛的官能团耐受性,可容纳供电子和吸电子取代基。该方法的多功能性突出表现在反应条件温和、反应时间短和克级可行性。最后,该方法可能为不对称催化打开大门,提供了获取对映体富集的邻苯二甲酸酯的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


