Zinc deposition characteristics in different electrolytes for aqueous zinc ion battery
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Aqueous zinc-ion batteries (ZIBs) are promising for sustainable energy storage due to high safety, low cost, and eco-friendliness, yet zinc metal anodes face challenges like dendrite growth, interfacial side reactions, and corrosion. To optimize electrolytes, this study systematically investigates five zinc salts (ZnSO4, Zn(ClO4)2, Zn(TFSI)2, Zn(NO3)2, and Zn(CH3COO)2) regarding zinc deposition behavior, interfacial stability, and electrochemical performance. Electrochemical techniques and multiscale characterizations reveal anion-influenced deposition and degradation mechanisms. ZnSO4 exhibited superior performance: symmetric cell cycled over 1700 h at 1 mA cm−2, Zn||Cu cell maintained 99.7 % of Coulombic efficiency (CE), and full cell delivered 76.7 mAh g−1 of average capacity. This stems from sulfate-induced formation of a stable Zn4SO4(OH)6·4H2O (ZHS) interfacial layer, suppressing dendrites. However, localized H2 evolution can raise pH and destabilize the interface. Zn(TFSI)2 showed low polarization and good stability at 5 mA cm−2, hydrophobic TFSI− may reduce surface impedance. Zn(ClO4)2 offered moderate stability, yet ClO4− reduction to corrosive Cl− limited long-term cycling. Conversely, Zn(CH3COO)2 and Zn(NO3)2 caused rapid decay: acetate decomposition produces corrosion-accelerating CO32−, while nitrate reduction generates acidic species inducing severe corrosion and short-circuit. This work offers critical insights into zinc electrolyte design, highlighting the key role of anion chemistry in interfacial engineering and cycling durability.
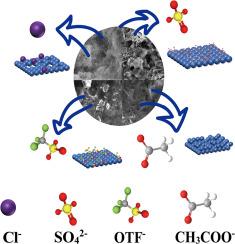
含水锌离子电池在不同电解质中的锌沉积特性
由于高安全性、低成本和生态友好性,水性锌离子电池(zbs)在可持续能源存储方面前景广阔,但锌金属阳极面临枝晶生长、界面副反应和腐蚀等挑战。为了优化电解质,本研究系统地研究了5种锌盐(ZnSO4、Zn(ClO4)2、Zn(TFSI)2、Zn(NO3)2和Zn(CH3COO)2)的锌沉积行为、界面稳定性和电化学性能。电化学技术和多尺度表征揭示了阴离子影响的沉积和降解机制。ZnSO4表现出优异的性能:对称电池在1 mA cm−2下循环超过2000 h, Zn||Cu电池保持99.7%的库仑效率(CE),满电池提供200 mAh g−1。这是由于硫酸盐诱导形成了稳定的Zn4SO4(OH)6·4H2O (ZHS)界面层,抑制了枝晶。然而,局部的H2演化会使pH升高,使界面不稳定。Zn(TFSI)2在5 mA cm−2下表现出低极化和良好的稳定性,但疏水性TFSI−可能会降低初始活性物质的利用率。Zn(ClO4)2具有中等稳定性,但ClO4−还原为腐蚀性Cl−限制了长期循环。相反,Zn(CH3COO)2和Zn(NO3)2导致快速衰变:乙酸分解产生加速腐蚀的CO32−,而硝酸盐还原产生酸性物质,导致严重腐蚀和短路。这项工作为锌电解质设计提供了重要的见解,突出了阴离子化学在界面工程和循环耐久性中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


