Proton transport mediated mesoporous SiO2 nanospheres for long-life lead-carbon battery
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
The development of carbon-based negative additives in lead-carbon batteries (LCBs) renders the premature failure of positive electrode constrains further life improvement of LCBs. In this study, mesoporous SiO2 nanospheres were prepared and incorporated into the positive electrode for promoting the cycling stability of LCBs. The action mechanism of SiO2 nanospheres in the positive electrode of LCBs was investigated via exploring the initial discharge capacity, fast charging ability, rate capability, and cycling performance. Mesoporous SiO2 nanospheres contribute to facilitate electrolyte penetration into the interior of positive active substances (PASs), while the hydroxyl groups on the surface and interior mesopores act as a proton reservoir, storing and releasing additional proton to promote the conversion kinetics of PbO2/PbSO4. Under the optimized content of 1.8 wt%, the highest initial discharge capacity of 3.36 Ah at 0.1 C and remarkable rate capability was achieved with a discharge capacity of 2.26 Ah at 1 C. Meanwhile, LCBs containing SiO2 maintained a capacity retention ratio of 124.5 % after 120 cycles, delivering prominent cycling stability. These favorable properties can be attributed to the synergistic effect of the mesoporous structure and hydroxyl groups of SiO2, which enhances the conversion rate of PASs and significantly improves the cycling stability.
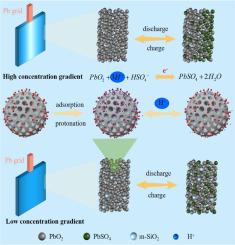
质子传输介导的中孔SiO2纳米球用于长寿命铅碳电池
碳基负极添加剂在铅碳电池中的应用,导致正极过早失效,制约了铅碳电池寿命的进一步提高。在本研究中,制备了介孔SiO2纳米球,并将其掺入正极中,以提高lcb的循环稳定性。通过对lcb正极初始放电容量、快速充电能力、倍率能力和循环性能的考察,研究了SiO2纳米球在lcb正极中的作用机理。介孔SiO2纳米球有助于电解质渗透到正活性物质(PASs)内部,而表面和内部介孔上的羟基充当质子库,储存和释放额外的质子,促进PbO2/PbSO4的转化动力学。在质量分数为1.8wt%的优化条件下,含SiO2的lcb在0.1 C条件下的初始放电容量达到3.36 Ah,在1 C条件下的放电容量达到2.26 Ah,具有显著的倍率性能。同时,含SiO2的lcb在120次循环后的容量保持率为124.5%,具有良好的循环稳定性。这些良好的性能可归因于SiO2的介孔结构和羟基的协同作用,提高了PASs的转化率,显著提高了循环稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


