Dynamic Interhalogen Coupling Engineered by Multifunctional Ionic Liquid for High-Energy Aqueous Zn-I2 Batteries
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aqueous Zn-I2 batteries are promising for sustainable energy storage, exhibit excellent safety, low cost, and high energy density. However, their practical application is limited by the two-electron I−/I⁰ redox reaction and the severe shuttle effect of polyiodide species. Here, a multifunctional ionic liquid, 1-butyl-3-methylimidazolium bromide (BmimBr), is introduced to establish a dynamic interhalogen coupling between iodine and bromine species, which enables a six-electron transfer pathway involving I−/I⁰/I⁺ and Br−/Br⁰. In situ characterizations and theoretical calculations show that Br− acts as a dynamic mediator, forming an [IBr2]− intermediate with iodine. This significantly lowers the energy barrier for oxidizing I⁰ to I⁺, thereby contributing additional capacity and accelerating kinetics. Simultaneously, Bmim⁺ provides dual interfacial functions: confining polyiodides at the cathode to suppress shuttling, and forming a protective layer on the zinc anode, which reduces interfacial water, inhibits dendrite growth and side reactions. Leveraging this synergistic design, the Zn-I2 battery achieves a high specific capacity of 511 mAh g−1 at 1 A g−1 and retains 90.22% of its capacity after 30 000 cycles at 10 A g−1. The dynamic interhalogen coupling strategy offers a novel route to activate multielectron reactions and stabilize electrode interfaces in Zn-I2 batteries.
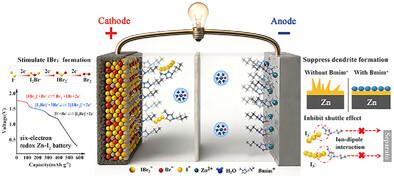
高能锌离子电池中多功能离子液体的动态卤素耦合研究
锌- i2水溶液电池具有良好的安全性、低成本和高能量密度等优点,是一种具有可持续能源存储前景的电池。然而,它们的实际应用受到双电子I - /I⁰氧化还原反应和多碘化物物种严重的穿梭效应的限制。本文引入了一种多功能离子液体,1-丁基-3-甲基咪唑溴(BmimBr),在碘和溴之间建立了动态卤素偶联,从而实现了一个涉及I - /I⁰/I⁺和Br - /Br⁰的六电子转移途径。原位表征和理论计算表明,Br−作为动态介质,与碘形成[IBr2]−中间体。这显著降低了I⁰氧化成I⁺的能垒,从而贡献了额外的容量和加速动力学。同时,Bmim⁺提供了双重界面功能:将多碘化物限制在阴极上抑制穿梭,在锌阳极上形成保护层,减少界面水分,抑制枝晶生长和副反应。利用这种协同设计,锌- i2电池在1ag - 1下实现了511 mAh g - 1的高比容量,并在10ag - 1下循环3万次后保持了90.22%的容量。动态卤素耦合策略为激活Zn-I2电池中的多电子反应和稳定电极界面提供了一条新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


