Efficient and Selective Ablation of Atheroma Using Watt-Level Mid-Infrared Femtosecond Laser at 5.75 µm
IF 10
1区 物理与天体物理
Q1 OPTICS
引用次数: 0
Abstract
Atherosclerotic cardiovascular disease remains a leading cause of global mortality. Despite advancements in pharmacological therapies and invasive interventions, including stenting and bypass surgery, current approaches are constrained by severe side effects, risks of restenosis and perioperative complications, significant recovery burdens, and high damage to vascular tissues. There is a critical need for a minimally invasive technique capable of selectively ablating atheromatous lesions while preserving healthy vascular tissues. In this study, leveraging the unique molecular composition of atherosclerotic plaques—rich in cholesteryl esters, and substantial spectral separation between the ester and artery absorption, selective laser ablation of atheroma is demonstrated. A 5.75 µm femtosecond laser system targeting the resonance of cholesteryl ester bonds, with a high power of 1.5 W is developed based on parametric amplifiers, to assess ablation efficiency and selectivity. The atherosclerotic plaques in a murine model of atherosclerosis are fully removed within milliseconds, while no damage to the artery is observed. Furthermore, the translational potential of this technology is validated by integrating a specially designed mid-infrared anti-resonant hollow-core fiber for efficient laser energy delivery, achieving an ablation rate of 0.13 mm3 s−1. These findings provide a promising strategy for rapid and selective intervention in atherosclerosis, with clear potential for clinical translation.
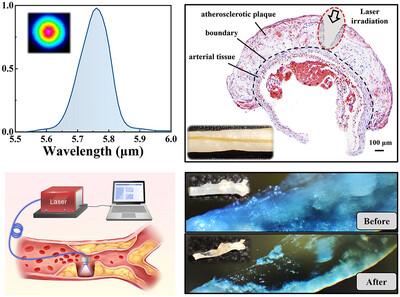
使用5.75µm的瓦级中红外飞秒激光有效和选择性消融动脉粥样硬化
动脉粥样硬化性心血管疾病仍然是全球死亡的主要原因。尽管药物治疗和侵入性干预(包括支架植入和搭桥手术)取得了进展,但目前的方法受到严重副作用、再狭窄风险和围手术期并发症、显著的恢复负担和对血管组织的高度损伤的限制。目前迫切需要一种微创技术,能够选择性地消融动脉粥样硬化病变,同时保留健康的血管组织。在这项研究中,利用动脉粥样硬化斑块独特的分子组成-富含胆固醇酯,以及酯和动脉吸收之间的大量光谱分离,证明了选择性激光消融动脉粥样硬化。在参数放大器的基础上,建立了一个5.75µm飞秒激光系统,以胆固醇酯键共振为目标,高功率为1.5 W,以评估烧蚀效率和选择性。在小鼠动脉粥样硬化模型中,动脉粥样硬化斑块在几毫秒内被完全去除,同时没有观察到动脉损伤。此外,通过集成专门设计的中红外抗谐振空心芯光纤,验证了该技术的转化潜力,该光纤可实现高效的激光能量传输,实现0.13 mm3 s−1的烧蚀速率。这些发现为动脉粥样硬化的快速和选择性干预提供了一个有希望的策略,具有明显的临床转化潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.20
自引率
5.50%
发文量
314
审稿时长
2 months
期刊介绍:
Laser & Photonics Reviews is a reputable journal that publishes high-quality Reviews, original Research Articles, and Perspectives in the field of photonics and optics. It covers both theoretical and experimental aspects, including recent groundbreaking research, specific advancements, and innovative applications.
As evidence of its impact and recognition, Laser & Photonics Reviews boasts a remarkable 2022 Impact Factor of 11.0, according to the Journal Citation Reports from Clarivate Analytics (2023). Moreover, it holds impressive rankings in the InCites Journal Citation Reports: in 2021, it was ranked 6th out of 101 in the field of Optics, 15th out of 161 in Applied Physics, and 12th out of 69 in Condensed Matter Physics.
The journal uses the ISSN numbers 1863-8880 for print and 1863-8899 for online publications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


