Clarifying the microscopic origin of Mn3+ ion instability in cathode oxides
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
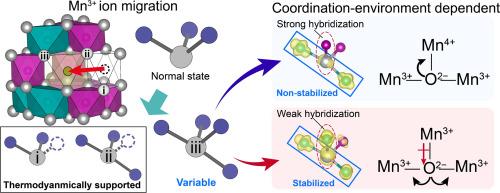
The pronounced mobility of Mn3+ ions in oxygen sublattice critically limits the utilization of Mn3+/Mn4+ redox couples for cathode materials. While Mn3+ instability has historically long been associated with their inherent Jahn-Teller (JT) effect, the microstructural features and electronic-state evolutions underlying the Mn migration remain insufficiently understood, hindering the development of effective Mn stabilization strategies. Here, we demonstrate that the Mn3+ site instability is not an inherent property of the JT effect but closely depends on their local coordination environment. Using spinel LiMn2O4 as a research model, we experimentally demonstrate that Mn migration induced by coordination instability preferentially occurs within the 0–50% SOC, where both Li vacancies and a high concentration of Mn3+ ions coexist. Under these structural conditions, weakly hybridized oxygen orbitals aligned with elongated Mn3+–O bonds act as electronic donors, stabilizing the linear Mn–O–Mn configuration in the degraded state. This electronic stabilization reduces the energetic penalty for Mn migration, thereby uncovering the microscopic origin of site instability that drives Mn3+ migration.

澄清阴极氧化物中Mn3+离子不稳定性的微观成因
Mn3+离子在氧亚晶格中明显的迁移性严重限制了Mn3+/Mn4+氧化还原偶对在正极材料中的应用。虽然Mn3+的不稳定性长期以来一直与其固有的Jahn-Teller (JT)效应有关,但Mn迁移背后的微观结构特征和电子态演变仍然没有得到充分的了解,这阻碍了有效的Mn稳定策略的发展。在这里,我们证明了Mn3+位点的不稳定性不是JT效应的固有性质,而是密切依赖于它们的局部配位环境。以尖晶石LiMn2O4为研究模型,实验证明了配位不稳定性导致的Mn迁移优先发生在0-50% SOC内,其中Li空位和高浓度Mn3+离子共存。在这些结构条件下,与细长的Mn3+ -O键排列的弱杂化氧轨道充当电子给体,稳定了降解状态下Mn-O-Mn的线性构型。这种电子稳定降低了Mn迁移的能量惩罚,从而揭示了驱动Mn3+迁移的位点不稳定性的微观起源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


