Inhibitory effect of an ethnoveterinary oil and water formulation on LTA-induced mastitis via TLR2/NF-κB signaling in mammary epithelial cells
IF 3.5
3区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Mastitis, an inflammatory disease, becoming a problematic condition in the dairy industry, which causes severe economic loss. It is primarily infected by Staphylococcus aureus (S. aureus). Current conventional therapies for mastitis often lead to antibiotics resistance. To address this issue, a plant based ethnoveterinary medicine approach is being explored as a potential treatment for this condition. The aim of the present study was to evaluate the anti-inflammatory and anti-mastitis effect of oil based (EO) and water based (EW) ethno veterinary formulations in Lipoteichoic acid (LTA) stimulated HC11 cells. LTA induced inflammatory model in HC11 mouse mammary epithelial cells were used in this study. The inflammatory genes expressions such as inducible nitric oxide synthase (iNOS), Toll like receptor 2 (TLR2), and NF-κB p65 were analysed by RT-PCR analysis. The antioxidant enzymes and inflammatory mediators were analysed using ELISA method. NAGase activity was analysed by using colorimetric method. Reactive oxygen species (ROS) generation was assessed by fluorescent microscopy method. Results revealed that EO inhibited the production of nitric oxide (NO), Lipid perioxidation and the gene expression of iNOS, TLR2, and NF-κB p65 in the LTA-induced HC11 cells. Moreover, the DCFH-DA staining showed that EO enhances the endogenous antioxidant enzymes activity and mitigated the oxidative stress and ROS generation. Also, results showed that the treatment with EO possesses potent anti-mastitis effect by inhibiting NAGase activity. These results indicated that EO exhibits its anti-inflammatory and anti-mastitis effects mainly through attenuating TLR2 and NF-κB signaling pathway. Hence, EO could be a potential therapeutic agent against mastitis.
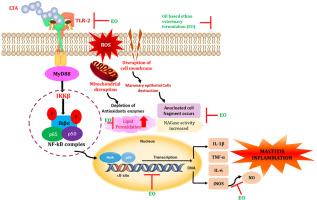
民族兽油和水配方通过TLR2/NF-κB信号传导抑制lta诱导的乳腺炎
乳腺炎,一种炎症性疾病,成为乳品行业的一个问题,造成严重的经济损失。它主要由金黄色葡萄球菌(金黄色葡萄球菌)感染。目前治疗乳腺炎的常规疗法常常导致抗生素耐药性。为了解决这个问题,正在探索一种基于植物的民族兽药方法作为治疗这种疾病的潜在方法。本研究的目的是评价油基(EO)和水基(EW)民族兽药制剂对脂质胆酸(LTA)刺激的HC11细胞的抗炎和抗乳腺炎作用。本研究采用LTA诱导的HC11小鼠乳腺上皮细胞炎症模型。采用RT-PCR分析诱导型一氧化氮合酶(iNOS)、Toll样受体2 (TLR2)、NF-κB p65等炎性基因的表达。采用ELISA法对抗氧化酶和炎症介质进行分析。用比色法分析NAGase活性。荧光显微镜法测定活性氧(ROS)的生成。结果显示,EO抑制了lta诱导的hc11细胞中一氧化氮(NO)的产生、脂质周氧化以及iNOS、TLR2和NF-κB p65的基因表达。DCFH-DA染色显示,EO增强了内源性抗氧化酶活性,减轻了氧化应激和ROS的产生。结果还表明,EO通过抑制NAGase活性,具有较强的抗乳腺炎作用。上述结果表明,EO主要通过抑制TLR2和NF-κB信号通路发挥抗炎、抗乳腺炎作用。因此,EO可能是一种潜在的乳腺炎治疗剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microbial pathogenesis
医学-免疫学
CiteScore
7.40
自引率
2.60%
发文量
472
审稿时长
56 days
期刊介绍:
Microbial Pathogenesis publishes original contributions and reviews about the molecular and cellular mechanisms of infectious diseases. It covers microbiology, host-pathogen interaction and immunology related to infectious agents, including bacteria, fungi, viruses and protozoa. It also accepts papers in the field of clinical microbiology, with the exception of case reports.
Research Areas Include:
-Pathogenesis
-Virulence factors
-Host susceptibility or resistance
-Immune mechanisms
-Identification, cloning and sequencing of relevant genes
-Genetic studies
-Viruses, prokaryotic organisms and protozoa
-Microbiota
-Systems biology related to infectious diseases
-Targets for vaccine design (pre-clinical studies)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


