YWHAG mediated epithelial-mesenchymal transition facilitates tumor progression and metastatic spread in non-small cell lung cancer
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Background
YWHAG, a 14–3-3 protein family member, has been implicated in tumorigenesis, yet its role in lung cancer progression and underlying mechanisms remain poorly understood. This study aimed to elucidate the functional and mechanistic contributions of YWHAG to non-small cell lung cancer (NSCLC).
Methods
Multi-omics analyses were performed using TCGA pan-cancer datasets, NSCLC single-cell RNA sequencing (scRNA-seq) data (GSE117570 & GSE148071), and public NSCLC mouse models. Functional validation included in vitro siRNA knockdown and overexpression of YWHAG in NCI-H292 cells, co-immunoprecipitation (Co-IP), immunofluorescence, and pathway activity assays. A lung-specific YWHAG knockout mouse model was generated using AAV5-SPC-Cre, and tumor growth/metastasis were assessed in an orthotopic A549 cell-derived NSCLC model via bioluminescence imaging and histopathology.
Results
YWHAG was significantly upregulated across 29 cancer types, including lung adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC), and correlated with poor patient survival. scRNA-seq revealed YWHAG-enriched proliferating epithelial cells (ECs) with elevated EMT activity (EMT_AUC score), marked by upregulation of COL12A1, FN1, and YWHAG. Mechanistically, YWHAG knockdown suppressed EMT (increased E-cadherin, decreased N-cadherin/Vimentin), proliferation, and migration, while overexpression exerted opposite effects. YWHAG interacted with LRRK2 to activate the PI3K/AKT pathway, and LRRK2 overexpression rescued PI3K/AKT suppression post-YWHAG knockdown. In mice, lung-specific YWHAG knockout attenuated tumor growth and metastasis, suppressed EMT markers, and reduced metastatic burden in lung, liver, and skeletal muscle.
Conclusion
YWHAG drives NSCLC progression by promoting EMT, proliferation, and metastasis via the LRRK2/PI3K/AKT axis. Its pan-cancer overexpression and prognostic significance highlights YWHAG as a promising therapeutic target in lung cancer.
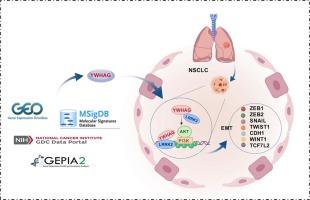
YWHAG介导的上皮-间质转化促进了非小细胞肺癌的肿瘤进展和转移扩散。
背景:14-3-3蛋白家族成员YWHAG与肿瘤发生有关,但其在肺癌进展中的作用及其潜在机制尚不清楚。本研究旨在阐明YWHAG在非小细胞肺癌(NSCLC)中的功能和机制贡献。方法:使用TCGA泛癌症数据集、NSCLC单细胞RNA测序(scRNA-seq)数据(GSE117570 & GSE148071)和公开的NSCLC小鼠模型进行多组学分析。功能验证包括体外siRNA敲除和YWHAG在NCI-H292细胞中的过表达、共免疫沉淀(Co-IP)、免疫荧光和途径活性测定。利用AAV5-SPC-Cre建立肺特异性YWHAG敲除小鼠模型,并通过生物发光成像和组织病理学评估原位A549细胞源性非小细胞肺癌模型的肿瘤生长/转移。结果:YWHAG在包括肺腺癌(LUAD)和鳞状细胞癌(LUSC)在内的29种癌症类型中显著上调,并与患者生存率低相关。scRNA-seq显示,YWHAG富集的增殖上皮细胞(ECs)具有升高的EMT活性(EMT_AUC评分),以COL12A1、FN1和YWHAG的上调为标志。在机制上,YWHAG敲低抑制EMT(增加E-cadherin,降低N-cadherin/Vimentin)、增殖和迁移,而过表达则发挥相反的作用。YWHAG与LRRK2相互作用激活PI3K/AKT通路,LRRK2过表达挽救了YWHAG敲低后PI3K/AKT的抑制。在小鼠中,肺特异性YWHAG敲除可减轻肿瘤生长和转移,抑制EMT标志物,并减少肺、肝脏和骨骼肌的转移负担。结论:YWHAG通过LRRK2/PI3K/AKT轴促进EMT、增殖和转移,从而驱动NSCLC的进展。其泛癌过表达和预后意义凸显了YWHAG作为肺癌治疗靶点的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


