LncRNA CTB-31O20.2 promotes the progression of hepatocellular carcinoma by targeting the miR-139-5p/ROCK1 axis
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hepatocellular carcinoma (HCC) is a primary cause of mortality from cancer, necessitating novel insights into its molecular underpinnings. Recent evidence points to the significant roles of lncRNAs in HCC progression, among which CTB-31O20.2 has emerged as a potentially crucial player. This study utilized RNA sequencing to identify differentially expressed lncRNAs in HCC tissues, focusing on CTB-31O20.2. We employed RT-qPCR, Western blotting, and dual-luciferase reporter assays to evaluate the expression patterns of CTB-31O20.2 in HCC cells and its interactions with miR-139-5p and Rho-associated coiled-coil containing protein kinase1 (ROCK1). In vivo effects were analyzed using a xenograft mouse model. CTB-31O20.2 was significantly upregulated in HCC tissues and cell lines. Silencing CTB-31O20.2 in Hep3B and HepG2 cells reduced malignancy, evidenced by decreased viability, colony formation, and invasion, and increased apoptosis. These cellular behaviors were associated with alterations in apoptosis-related proteins and epithelial-mesenchymal transition (EMT) markers. CTB-31O20.2 was shown to interact with miR-139-5p, and its silencing upregulated miR-139-5p, leading to reduced ROCK1 expression. Conversely, miR-139-5p inhibition reversed the anti-tumor effects of CTB-31O20.2 silencing. Overexpression of ROCK1 negated the inhibitory effects on HCC cell malignancy induced by CTB-31O20.2 depletion. In vivo, CTB-31O20.2 silencing significantly reduced tumor growth in a xenograft model. Our findings reveal that CTB-31O20.2 contributes to HCC progression by modulating miR-139-5p and ROCK1 expression. These results highlight CTB-31O20.2 as a potential therapeutic target in HCC, providing a new perspective on the mechanisms driving HCC pathogenesis and progression.
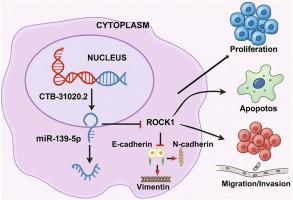
LncRNA CTB-31O20.2通过靶向miR-139-5p/ROCK1轴促进肝细胞癌的进展。
肝细胞癌(HCC)是癌症死亡的主要原因,需要对其分子基础进行新的见解。最近的证据指出lncrna在HCC进展中的重要作用,其中ctb - 31020.2已成为一个潜在的关键角色。本研究利用RNA测序技术鉴定HCC组织中差异表达的lncRNAs,重点关注CTB-31O20.2。我们采用RT-qPCR、Western blotting和双荧光素酶报告基因检测来评估ccb - 31o20.2在HCC细胞中的表达模式及其与miR-139-5p和rho相关的卷曲卷曲含蛋白激酶1 (ROCK1)的相互作用。使用异种移植小鼠模型分析体内效应。CTB-31O20.2在HCC组织和细胞系中显著上调。在Hep3B和HepG2细胞中沉默CTB-31O20.2可减少恶性肿瘤,表现为细胞活力、集落形成和侵袭降低,以及细胞凋亡增加。这些细胞行为与凋亡相关蛋白和上皮-间质转化(EMT)标记物的改变有关。CTB-31O20.2被证明与miR-139-5p相互作用,其沉默上调miR-139-5p,导致ROCK1表达降低。相反,miR-139-5p抑制逆转了CTB-31O20.2沉默的抗肿瘤作用。ROCK1过表达对CTB-31O20.2缺失诱导的HCC细胞恶性肿瘤的抑制作用无效。在体内,CTB-31O20.2沉默可显著降低异种移植物模型中的肿瘤生长。我们的研究结果表明,CTB-31O20.2通过调节miR-139-5p和ROCK1的表达来促进HCC的进展。这些结果突出了CTB-31O20.2作为HCC的潜在治疗靶点,为HCC的发病和进展机制提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


