Radiobiological effects of AB-BNCT on tongue squamous cell carcinoma: An in vitro and in vivo study
IF 1.8
3区 工程技术
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
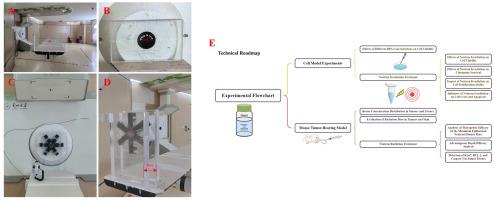
This study systematically investigated the radiobiological effects of an accelerator-based boron neutron capture therapy (AB-BNCT) system on SAS cells, a human tongue squamous cell carcinoma (TSCC) cell line. In vitro experimental results showed that L-10B-BPA incubation within 12 h had no significant effect on cell viability. At 24 and 48 h post-irradiation, the 26N20 group exhibited 11 % and 31.91 % reductions in cell viability, accompanied by 82.15 % and 68.34 % decreases in proliferation rates, respectively, compared to controls. In the 26N10 group, 48-h post-irradiation analysis revealed 49.05 % G2/M phase cell cycle arrest and a 40.17 % apoptosis rate (p < 0.05).
In vivo experiments revealed that 1 h after L-10B-BPA administration, the boron concentration in tumor tissue reached 59.36 ppm with tumor-to-normal tissue (T/N) ratio reached a maximum value of 8.48. Thirteen days after treatment, a complete tumor response was observed in 33 % of cases, while the objective response rate reached 100 %. Median survival time was significantly prolonged to 68 days from 27days for control (p < 0.05). Notably, at advantage depth, the L-10B-BPA combined with neutron irradiation failed to confer survival benefit. Neutron irradiation alone demonstrated tumor growth-promoting effects at identical fluence levels. These findings underscore the critical need to address dose cold spots in deep-seated tumors during clinical applications.
These results indicate that the AB-BNCT system effectively suppresses the progression of TSCC, likely through the induction of DNA damage and apoptosis. Further studies will explore the underlying molecular mechanisms of BNCT-induced apoptosis, providing a scientific basis for its precise clinical application.
AB-BNCT对舌鳞状细胞癌的放射生物学作用:体外和体内研究。
本研究系统地研究了基于加速器的硼中子俘获治疗(AB-BNCT)系统对人舌鳞癌(TSCC)细胞系SAS细胞的放射生物学效应。体外实验结果表明,L-10B-BPA孵育12 h内对细胞活力无显著影响。在照射后24和48 h, 26N20组细胞活力分别下降11%和31.91%,增殖率分别下降82.15%和68.34%。26N10组照射48 h后,细胞G2/M期周期阻滞49.05%,凋亡率40.17% (p 10B-BPA),肿瘤组织中硼浓度达到59.36 ppm,肿瘤与正常组织(T/N)比达到最大值8.48。治疗13天后,33%的病例肿瘤完全缓解,客观缓解率达到100%。中位生存时间从对照组的27天显著延长至68天(p 10B-BPA联合中子照射未能获得生存益处)。单独的中子辐照在相同的剂量水平下显示出促进肿瘤生长的作用。这些发现强调了在临床应用中解决深部肿瘤剂量冷点的迫切需要。这些结果表明,AB-BNCT系统可能通过诱导DNA损伤和细胞凋亡有效抑制TSCC的进展。进一步的研究将探索bnct诱导细胞凋亡的分子机制,为其精确的临床应用提供科学依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Radiation and Isotopes
工程技术-核科学技术
CiteScore
3.00
自引率
12.50%
发文量
406
审稿时长
13.5 months
期刊介绍:
Applied Radiation and Isotopes provides a high quality medium for the publication of substantial, original and scientific and technological papers on the development and peaceful application of nuclear, radiation and radionuclide techniques in chemistry, physics, biochemistry, biology, medicine, security, engineering and in the earth, planetary and environmental sciences, all including dosimetry. Nuclear techniques are defined in the broadest sense and both experimental and theoretical papers are welcome. They include the development and use of α- and β-particles, X-rays and γ-rays, neutrons and other nuclear particles and radiations from all sources, including radionuclides, synchrotron sources, cyclotrons and reactors and from the natural environment.
The journal aims to publish papers with significance to an international audience, containing substantial novelty and scientific impact. The Editors reserve the rights to reject, with or without external review, papers that do not meet these criteria.
Papers dealing with radiation processing, i.e., where radiation is used to bring about a biological, chemical or physical change in a material, should be directed to our sister journal Radiation Physics and Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


