IL-33 links inflammation and bone remodeling in experimental spondyloarthritis and human joint biopsies
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Objective
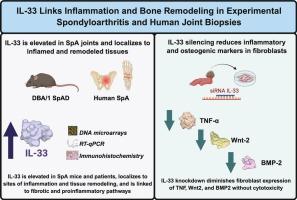
To investigate the pathogenic role of IL-33 in spondyloarthritis (SpA) by analyzing its expression in both murine and human joint tissues and functionally assessing its impact on fibroblasts. The study evaluated synovial and entheseal biopsies from patients with SpA to explore the potential contribution of IL-33 to joint inflammation and tissue remodeling.
Methods
A spontaneous arthritis model (SpAD) in DBA/1 mice was used to assess IL-33 expression via histology, immunohistochemistry, transcriptomics, RT-qPCR, and western blot. Sacroiliac and tarsal biopsies from patients with SpA were also analyzed. Differential gene expression was checked, and pathway enrichment was performed using Ingenuity Pathway Analysis. Primary fibroblasts were isolated from the joints of the mice's front and rear limbs, transfected with siRNA targeting Il33, and evaluated by RT-qPCR and western blot for inflammatory (Tnf, Nfkb) and osteogenic (Wnt2, Bmp2) markers. Cell viability was assessed via MTT assay.
Results
IL-33 expression was elevated in murine and human SpA joints, with strong cartilage and subchondral bone localization. Transcriptomic data indicated upregulation of IL-33 signaling and enrichment of proinflammatory and fibrotic pathways. Silencing of Il33 in fibroblasts significantly reduced IL-33 protein levels and decreased Tnf and Wnt2 expression at both mRNA and protein levels, while Bmp2 reduction was observed only at the transcript level.
Conclusion
IL-33 contributes to joint inflammation and may regulate osteogenic pathways implicated in pathological bone formation. These findings support IL-33 as a potential dual-action therapeutic target in SpA.
IL-33与实验性脊柱炎和人类关节活检中的炎症和骨重塑有关。
目的:通过分析IL-33在小鼠和人关节组织中的表达及对成纤维细胞的功能影响,探讨IL-33在脊椎关节炎(SpA)中的致病作用。该研究评估了SpA患者的滑膜和骨膜活检,以探索IL-33对关节炎症和组织重塑的潜在贡献。方法:采用组织学、免疫组化、转录组学、RT-qPCR和western blot检测DBA/1小鼠自发性关节炎模型(SpAD)中IL-33的表达。还分析了SpA患者的骶髂和跗骨活检。检测差异基因表达,并使用Ingenuity pathway Analysis进行途径富集。从小鼠前肢和后肢关节分离原代成纤维细胞,转染靶向Il33的siRNA,并通过RT-qPCR和western blot检测炎症(Tnf, Nfkb)和成骨(Wnt2, Bmp2)标志物。MTT法测定细胞活力。结果:IL-33在小鼠和人SpA关节中表达升高,具有较强的软骨和软骨下骨定位。转录组学数据显示IL-33信号的上调以及促炎和纤维化途径的富集。在成纤维细胞中沉默Il33可显著降低IL-33蛋白水平,并在mRNA和蛋白水平上降低Tnf和Wnt2的表达,而仅在转录水平上观察到Bmp2的降低。结论:IL-33参与关节炎症,并可能调控病理性骨形成的成骨途径。这些发现支持IL-33作为SpA的潜在双作用治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


