Advancing antisense oligonucleotide delivery through click chemistry based chemical conjugation with designed short non-cationic peptides for Duchenne muscular dystrophy
IF 4.3
3区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Duchenne muscular dystrophy (DMD) is a fatal X-linked neuromuscular disease caused by frame shift mutations in the gene encoding dystrophin. 2́-O-methyl phosphorothioate (2′-OMePS) serves as an antisense RNA platform clinically used in DMD patients to facilitate exon skipping and production of an internally truncated, yet functional dystrophin protein. Effective delivery and uptake of antisense oligonucleotides (ASOs) by target cells are crucial for their efficacy. Peptide-conjugated ASOs offer a promising next-generation platform, where a cell-penetrating peptide (CPP) is linked to the 2′-OMePS backbone to enhance cellular uptake. Herein, we designed and synthesized a new non-cationic short CPP sequence that can be efficiently conjugated with the negatively charged 2′-OMePS ASO backbone using click chemistry. Conjugation of the lead peptide ETWWK to 2′-OMePS ASO resulted in significant cellular internalization with precise nuclear localization of the ASO cargo. Cellular uptake was assessed in C2C12 and human DMD patient-derived myoblast cells via fluorescence microscopy and flow cytometry. Additionally, the synthesized ETWWK-ASO conjugate exhibits a significant 1.94 fold upregulation of dystrophin protein in the clinically relevant DMD patient-derived cell line. Our findings suggest that the identified peptide holds promise for facilitating ASO delivery at the site of splicing. This study highlights the efficient conjugation of CPPs to negatively charged 2′-OMePS ASO through tailored conjugation strategies, and will eventually be a therapeutic avenue for future ASO-based DMD treatments.
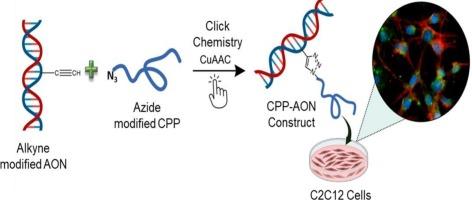
通过点击化学与设计的短非阳离子肽的化学偶联,推进反义寡核苷酸递送治疗杜氏肌营养不良。
杜氏肌营养不良症(DMD)是一种致命的x连锁神经肌肉疾病,由编码肌营养不良蛋白的基因的框架移位突变引起。2′- o -甲基磷硫酸酯(2′-OMePS)作为一种反义RNA平台,在临床上用于DMD患者,以促进外显子跳变和产生内部截断但具有功能的肌营养不良蛋白。反义寡核苷酸(ASOs)通过靶细胞的有效递送和摄取是其有效性的关键。肽偶联ASOs提供了一个有前途的下一代平台,其中细胞穿透肽(CPP)连接到2'-OMePS主干以增强细胞摄取。在此,我们设计并合成了一个新的非阳离子短CPP序列,该序列可以有效地与带负电荷的2'-OMePS ASO主链结合。将先导肽ETWWK偶联到2'-OMePS ASO上,通过对ASO货物进行精确的核定位,实现了显著的细胞内化。通过荧光显微镜和流式细胞术评估C2C12和人DMD患者来源的成肌细胞的细胞摄取。此外,合成的ETWWK-ASO偶联物在临床相关的DMD患者来源的细胞系中显示出1.94倍的肌营养不良蛋白上调。我们的研究结果表明,鉴定的肽有望促进剪接位点的ASO传递。该研究强调了通过定制的偶联策略将CPPs与带负电荷的2'-OMePS ASO有效偶联,并最终将成为未来基于ASO的DMD治疗的治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Methods
生物-生化研究方法
CiteScore
9.80
自引率
2.10%
发文量
222
审稿时长
11.3 weeks
期刊介绍:
Methods focuses on rapidly developing techniques in the experimental biological and medical sciences.
Each topical issue, organized by a guest editor who is an expert in the area covered, consists solely of invited quality articles by specialist authors, many of them reviews. Issues are devoted to specific technical approaches with emphasis on clear detailed descriptions of protocols that allow them to be reproduced easily. The background information provided enables researchers to understand the principles underlying the methods; other helpful sections include comparisons of alternative methods giving the advantages and disadvantages of particular methods, guidance on avoiding potential pitfalls, and suggestions for troubleshooting.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


