Investigation of Pd Catalyst for Key Coupling Reaction in Asciminib Synthesis and Impurity Characterization
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
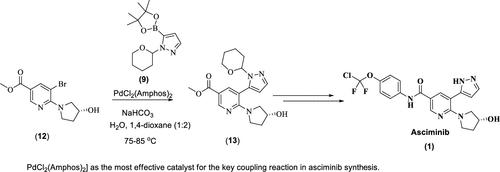
Asciminib, marketed as Scemblix, is approved for treating adults with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP), as well as for those previously treated with two or more tyrosine kinase inhibitors (TKIs). Existing literature on Asciminib manufacturing processes involves considerable quantities of preformed Pd complex catalysts for the key coupling reaction. We optimized the commercial production process by identifying dichlorobis[ditert-butyl(4-dimethylaminophenyl)phosphine]palladium(II) PdCl2(Amphos)2 as the most effective catalyst for the key coupling reaction. By fine-tuning the Pd complex quantity to a 0.0035 weight ratio relative to the key starting material─approximately one-third of the previously reported amount─we achieved complete conversion and obtained the desired coupling product in high yield. Additionally, we developed a highly effective and commercially viable method combining l-cystine and activated carbon to efficiently remove Pd metal impurities from the final product. The optimized process was successfully validated under GMP conditions at the multikilogram level and has proven to be easily scalable for larger quantities. This report also addresses the lack of detailed information on potential process and degradation impurities of Asciminib. We identified and synthesized potential process impurities (P-1 to P-5) and degradation impurities (DP-1 to DP-3), confirming their structures using 1H NMR, 13C NMR, mass spectrometry, and IR spectroscopy. This comprehensive impurity identification enhances the quality and safety of Asciminib and supports the development of robust analytical methods for impurity quantification and validation.
阿西米尼合成关键偶联反应钯催化剂的研究及杂质表征
Asciminib以Scemblix的名称上市,被批准用于治疗新诊断的费城染色体阳性慢性髓性白血病(Ph+ CML)慢行期(CP)的成人患者,以及先前接受两种或两种以上酪氨酸激酶抑制剂(TKIs)治疗的患者。关于阿西米尼制造工艺的现有文献涉及大量用于关键偶联反应的预成形Pd络合物催化剂。通过确定二氯[二叔丁基(4-二甲氨基苯基)膦]钯(II) PdCl2(Amphos)2为关键偶联反应最有效的催化剂,对商业化生产工艺进行了优化。通过将钯络合物的数量微调到与关键原料的重量比为0.0035(约为先前报道量的三分之一),我们实现了完全转化,并获得了期望的高收率偶联产物。此外,我们开发了一种高效且商业可行的方法,将l-胱氨酸和活性炭结合起来,有效地去除最终产品中的钯金属杂质。优化后的工艺在GMP条件下成功地进行了多公斤级的验证,并且很容易扩展到更大的批量。本报告还解决了阿西米尼的潜在过程和降解杂质的详细信息的缺乏。我们鉴定并合成了潜在的工艺杂质(P-1至P-5)和降解杂质(DP-1至DP-3),并通过1H NMR、13C NMR、质谱和IR光谱确定了它们的结构。这种全面的杂质鉴定提高了阿西米尼的质量和安全性,并支持开发可靠的杂质定量和验证分析方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


