Multiple-reaction kinetics of composite electrodes for sulfide-based all-solid-state batteries: Impedance decoupling
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
All-solid-state batteries (ASSBs) have garnered considerable attention as next-generation energy-storage systems owing to their safety. In ASSBs, composite electrodes comprising solid electrolytes, active materials, and conductive additives are employed to increase the electrochemically active surface and ensure the Li+/electron pathways. Composite electrodes consist of numerous solid–solid contact interfaces and exhibit interfacial side reactions that inhibit charge transfer. Thus, a comprehensive analysis of their electrochemical processes is necessary to develop high-performance ASSBs. Herein, we demonstrate the quantitative analyses of the multiple-reaction kinetics in a solid composite electrode for ASSBs with state-of-the-art Li6PS5Cl electrolytes and LiNi0.8Co0.1Mn0.1O2 active materials using modified transmission line models based on networks of distributed impedance elements. Firstly, model simulations are conducted with different boundary conditions and circuit elements to investigate the impedance responses of electrodes. The circuit elements represent the interfacial and charge/atom transport processes, namely, Li+/electron transport, charge transfer, double-layer charging, and diffusion in the active material. Secondly, for quantitative experimental analyses, two model cells (i.e., symmetric cells and full cells) are fabricated with various electrode formulations, and their impedance spectra are measured as a function of temperature. Kinetic parameters are determined using the appropriate equivalent circuit models to elucidate the effects of electrode formulation and operating conditions on electrochemical performance. Finally, the interplay between the kinetics of ionic transport and interfacial reaction is discussed based on measurements using uncoated active materials and halide electrolytes. Thus, this study affords insights into the electrochemical processes of ASSBs and guidelines for quantitative analyses via the impedance decoupling method.
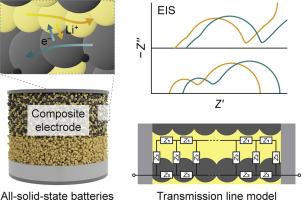
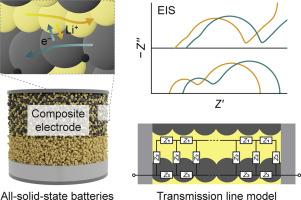
硫化物基全固态电池复合电极的多反应动力学:阻抗解耦
全固态电池(assb)作为下一代储能系统因其安全性而备受关注。在assb中,使用由固体电解质、活性材料和导电添加剂组成的复合电极来增加电化学活性表面并确保Li+/电子路径。复合电极由许多固体-固体接触界面组成,并表现出抑制电荷转移的界面副反应。因此,有必要对其电化学过程进行全面分析,以开发高性能的assb。本文采用基于分布阻抗单元网络的改进传输线模型,对采用最先进的Li6PS5Cl电解质和LiNi0.8Co0.1Mn0.1O2活性物质的assb固体复合电极中的多重反应动力学进行了定量分析。首先,采用不同的边界条件和电路元件进行了模型仿真,研究了电极的阻抗响应。电路元件代表界面和电荷/原子输运过程,即Li+/电子输运、电荷转移、双层充电和活性材料中的扩散。其次,在定量实验分析中,用不同的电极配方制作了两个模型电池(即对称电池和满电池),并测量了它们的阻抗谱作为温度的函数。采用适当的等效电路模型确定了动力学参数,以阐明电极配方和操作条件对电化学性能的影响。最后,讨论了离子传递动力学和界面反应之间的相互作用,基于测量使用未包覆的活性材料和卤化物电解质。因此,本研究为assb的电化学过程提供了见解,并为通过阻抗解耦方法进行定量分析提供了指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


