Supramolecular-Engineered Immune Modulator Orchestrates Tumor Microenvironment Reprogramming for Antibody-Independent Radio-Immunotherapy
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cancer immunotherapy has revolutionized oncological treatment by harnessing the immune system to target and eliminate malignant cells, with radiotherapy (RT) distinguished among immune-activating strategies for its potent ability to trigger immunogenic cell death (ICD). However, the antitumor immune responses by RT-mediated ICD are frequently hindered by the RT resistance and immunosuppressive tumor microenvironment (TME), particularly sustained hypoxia and cytoprotective autophagy. Herein, a supramolecular immune modulator based on sulfonated azocalix[4]arene (SAC4A)-engineered natural catalase (CAT) with hydroxychloroquine (HCQ) encapsulation to potentiate antibody-independent radio-immunotherapy by reprogramming TME is developed. This designed modulator can alleviate tumor hypoxic microenvironment and overcome hypoxia-induced RT resistance by converting tumor endogenous hydrogen peroxide (H2O2) into oxygen (O2). Simultaneously, the released HCQ from SAC4A-engineered CAT under the catalysis of tumor-overexpressed azo reductase can disrupt the autophagy process, further reprogramming the immunosuppressive TME and amplifying the RT-induced ICD effect. As a result, treatment with this supramolecular-engineered immune modulator can significantly enhance the therapeutic efficacy of RT against 4T1 tumor by evoking robust antitumor immune responses, including promotion of dendritic cells maturation, cytotoxic T lymphocytes infiltration, and M1-like macrophages repolarization. Therefore, this work provides a simple and efficient supramolecular engineering strategy to modulate TME and enhance radio-immunotherapy, greatly promising for clinical translation.
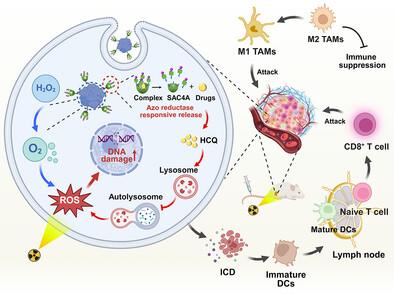
超分子工程免疫调节剂协调肿瘤微环境重编程,用于不依赖抗体的放射免疫治疗
通过利用免疫系统靶向和消除恶性细胞,癌症免疫治疗已经彻底改变了肿瘤治疗,放射治疗(RT)因其触发免疫原性细胞死亡(ICD)的强大能力而在免疫激活策略中脱颖而出。然而,RT介导的ICD的抗肿瘤免疫应答经常受到RT抵抗和免疫抑制性肿瘤微环境(TME),特别是持续缺氧和细胞保护性自噬的阻碍。本文开发了一种基于磺化偶氮杂环[4]arene (SAC4A)工程天然过氧化氢酶(CAT)和羟氯喹(HCQ)包封的超分子免疫调节剂,通过重编程TME来增强抗体非依赖性放射免疫治疗。该调节剂通过将肿瘤内源性过氧化氢(H2O2)转化为氧气(O2),缓解肿瘤缺氧微环境,克服缺氧诱导的RT抗性。同时,在肿瘤过表达的偶氮还原酶的催化下,sac4a工程CAT释放的HCQ可以破坏自噬过程,进一步重编程免疫抑制TME,放大rt诱导的ICD效应。因此,使用这种超分子工程免疫调节剂治疗可以通过激发强大的抗肿瘤免疫反应,包括促进树突状细胞成熟、细胞毒性T淋巴细胞浸润和m1样巨噬细胞复极化,显著提高RT对4T1肿瘤的治疗效果。因此,这项工作提供了一种简单有效的超分子工程策略来调节TME和增强放射免疫治疗,具有很大的临床应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


