Molten salt electrodeposition enabling direct and efficient preparation of homogeneous Pb-Li eutectic alloys
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Liquid lead-lithium (Pb-Li) alloys are promising tritium breeder materials for nuclear fusion, but conventional high-temperature smelting preparation process remains hampered by low efficiency, compositional segregation, and safety risks from metallic Li feedstocks. Herein, we propose a molten salt electrodeposition strategy using a liquid Pb metal electrode to directly produce Pb-Li alloys. Driven by in-situ alloying and depolarization effects, Li+ ions from LiCl-KCl molten salts undergo underpotential deposition on the liquid Pb cathode, which enhances current efficiency and reduces electrolysis energy consumption. Homogeneous Pb-Li alloys are stably obtained across a wide range of conditions, including Li content (10–35 at%), current density (100–500 mA·cm⁻²), and temperature (400–550 °C), with current efficiencies consistently maintained above 80 %. A kilogram-scale experiment validates that potential-feedback galvanostatic electrodeposition enables precise synthesis of Pb-Li eutectic alloys with an average Li content of 17.10 ± 0.80 at %, reducing carbon emissions by 83.9 kg CO₂e·t⁻¹-alloy and lowering production costs by $143.6 per ton alloy compared to smelting. This approach exhibits stability, sustainability, and scalability for precisely fabricating homogeneous Pb-Li and analogous Li-based alloys.

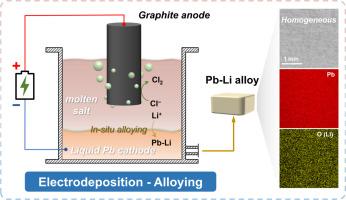
熔盐电沉积可以直接和有效地制备均匀的铅-锂共晶合金
液态铅锂(Pb-Li)合金是一种很有前途的核聚变氚增殖材料,但传统的高温熔炼制备工艺仍然存在效率低、成分偏析和金属Li原料安全风险等问题。在此,我们提出了一种使用液态铅金属电极的熔盐电沉积策略来直接生产铅锂合金。在原位合金化和去极化效应的驱动下,LiCl-KCl熔盐中的Li +离子在液态Pb阴极上进行欠电位沉积,提高了电流效率,降低了电解能耗。均匀的铅-锂合金可以在各种条件下稳定地获得,包括Li含量(10-35 %),电流密度(100-500 mA·cm⁻²)和温度(400-550°C),电流效率始终保持在80%以上。千克级实验证实,电位反馈恒流电沉积可以精确合成平均锂含量为17.10±0.80 at%的Pb-Li共晶合金,减少83.9 kg CO₂e·t -¹合金的碳排放,与冶炼相比,每吨合金的生产成本降低149.30美元。这种方法具有稳定性、可持续性和可扩展性,可用于精确制造均匀的铅-锂和类似的锂基合金。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


