Iron tuned Ni–Co–Fe alloy films via electrodeposition for hydrogen evolution reaction
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Compositionally tuned Ni-Co-Fe alloy films with iron (Fe) concentration from 1 to 14 at% are electrodeposited on stainless steel. The effect of iron addition to Ni-Co alloy on electrocatalytic hydrogen evolution reaction (HER) is studied in 1 M KOH. Iron (Fe) is incorporated to lower the nickel (Ni) content from ∼45 at% to ∼31 at% in Ni-Co alloys, resulting in notable changes in structural, morphological, and electronic properties. Structural and morphological analysis confirm that the Ni-Co-Fe (Fe = 4 at%) alloy film possesses the highest crystallinity with a larger grain size among the other electrodeposited alloy films. Fe appears in mixed valence states (Fe²⁺/Fe³⁺), while shifts in Ni 2p and Co 2p binding energies suggest strong electronic interactions and enhanced charge delocalization. The Ni-Co-Fe alloy film with 4 at% of iron demonstrates better electrocatalytic performance with a lower overpotential value than other compositions. The Ni-Co-Fe (Fe = 4 at%) alloy film exhibits the lowest charge transfer resistance, along with superior electrochemical surface area, roughness, and wettability among the electrodeposited alloy films. The Ni-Co-Fe alloy film with 4 at% Fe exhibits stable performance toward the hydrogen evolution reaction (HER). Thus, iron (Fe) can effectively substitute a significant amount of nickel (Ni) in Ni-Co alloys, enabling the development of cost-effective HER catalysts.
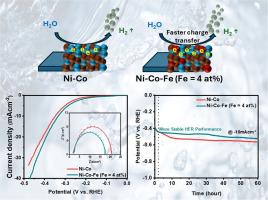
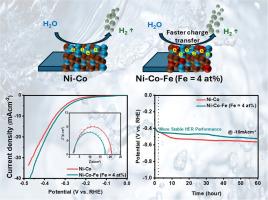
电沉积铁调谐Ni-Co-Fe合金析氢膜
在不锈钢上电沉积了铁(Fe)浓度为1 ~ 14at %的Ni-Co-Fe合金膜。研究了Ni-Co合金在1M KOH条件下加入铁对电催化析氢反应(HER)的影响。在Ni- co合金中加入铁(Fe)使镍(Ni)含量从~ 45at %降低到~ 31at %,导致结构、形态和电子性能的显著变化。结构和形态分析表明,Ni-Co-Fe (Fe = 4 at%)合金薄膜结晶度最高,晶粒尺寸较大。Fe以混合价态出现(Fe²+ /Fe³+),而Ni 2p和Co 2p结合能的变化表明电子相互作用强,电荷离域增强。铁含量为4 at%的Ni-Co-Fe合金膜具有较好的电催化性能和较低的过电位值。Ni-Co-Fe (Fe = 4 at%)合金膜表现出最低的电荷转移电阻,并具有优异的电化学表面积、粗糙度和润湿性。含4 at% Fe的Ni-Co-Fe合金膜在析氢反应(HER)中表现出稳定的性能。因此,铁(Fe)可以有效地替代Ni- co合金中的大量镍(Ni),从而开发出具有成本效益的HER催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


