Gangliosides modulate the secretion of extracellular vesicles and their misfolded protein cargo
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
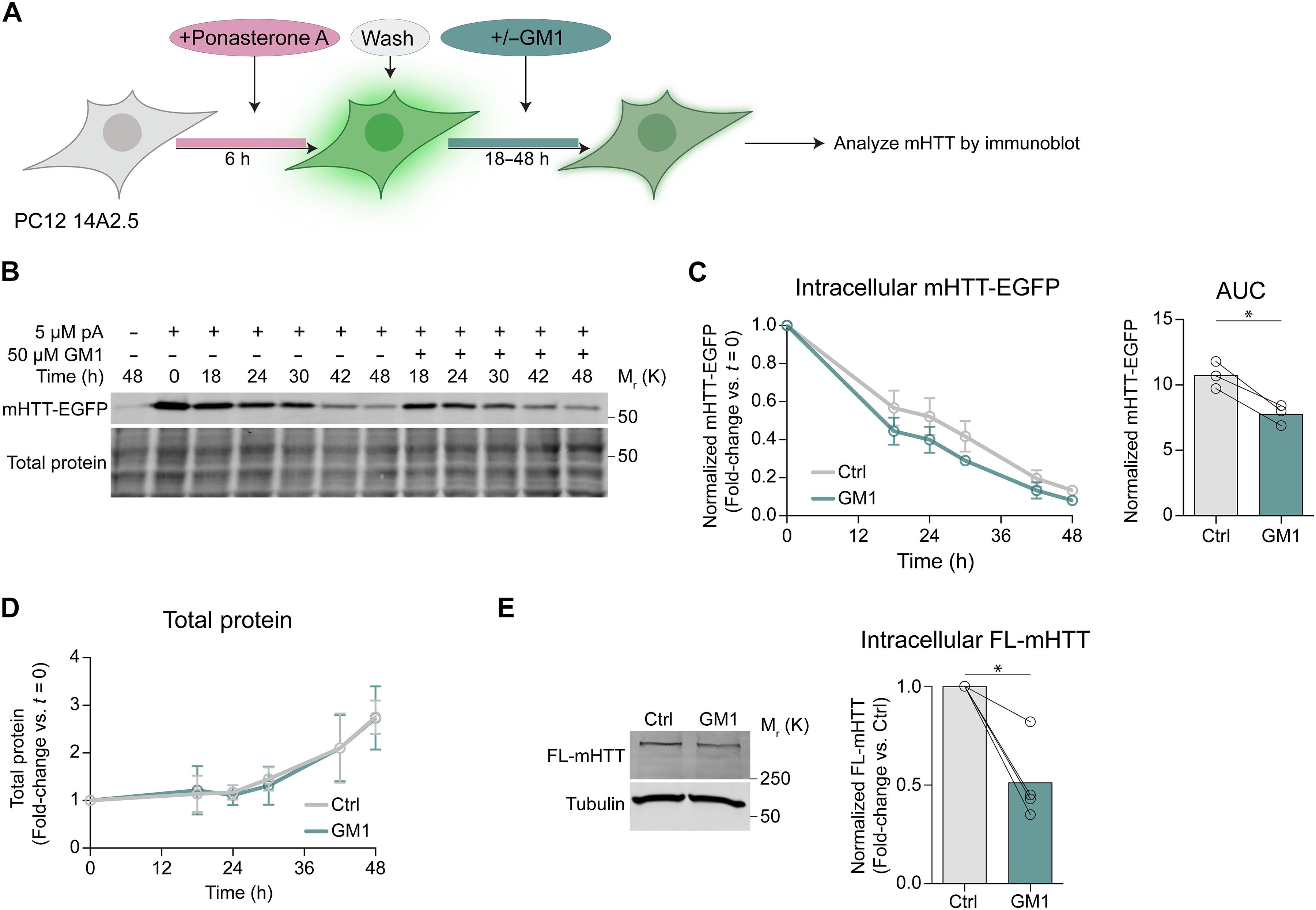
Gangliosides are glycosphingolipids with important roles in cell signaling and neuroprotection. While present on extracellular vesicles (EVs)—key mediators of intercellular communication—their role in EV biogenesis remains unclear. Here, we identify gangliosides as key modulators of EV biogenesis, with the specific composition of their glycan headgroup and the presence or absence of sialic acid and N-acetyl-d-galactosamine residues dictating whether they promote or inhibit EV biogenesis. GM1 and other complex gangliosides enhance EV secretion, while disruption of ganglioside synthesis impairs it. GM1 supplementation restores EV secretion in Huntington’s disease (HD) fibroblasts and cell models with ganglioside deficiency, including models of neurodegenerative diseases caused by a genetic block of ganglioside synthesis. Notably, GM1 enhances EV-mediated secretion of pathogenic misfolded proteins, including mutant huntingtin (mHTT), α-synuclein, and tau, reducing intracellular burden and providing mechanistic insight into the mHTT-lowering effects of GM1 in HD models. Our findings shed light on the neuroprotective roles of gangliosides and their therapeutic potential in misfolded protein disorders.
神经节苷调节细胞外囊泡及其错误折叠的蛋白质货物的分泌
神经节苷脂是鞘糖脂,在细胞信号传导和神经保护中起重要作用。虽然它们存在于细胞外囊泡(EV)中,这是细胞间通讯的关键介质,但它们在EV生物发生中的作用尚不清楚。在这里,我们确定神经节苷脂是EV生物发生的关键调节剂,其聚糖头基团的特定组成以及唾液酸和N -乙酰基- d -半乳糖胺残基的存在与否决定了它们是促进还是抑制EV生物发生。GM1等复杂神经节苷能促进EV的分泌,而破坏神经节苷的合成则会损害EV的分泌。补充GM1可恢复亨廷顿氏病(HD)成纤维细胞和神经节苷脂缺乏的细胞模型(包括由神经节苷脂合成遗传阻滞引起的神经退行性疾病模型)中EV的分泌。值得注意的是,GM1增强了eve介导的致病性错误折叠蛋白的分泌,包括突变型亨廷顿蛋白(mHTT)、α-突触核蛋白和tau蛋白,减少了细胞内负担,并为GM1在HD模型中降低mHTT的作用提供了机制。我们的发现揭示了神经节苷脂的神经保护作用及其在错误折叠蛋白质疾病中的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


