Living ROMP of poly(m,p-phenylenevinylene) and functionalized norbornene-dicarboximides copolymers: guided synthesis toward enhanced optoelectronic and thermal properties with DFT insights
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
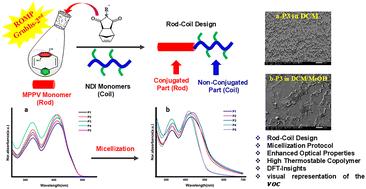
A series of functionalized copolymers is synthesized via ring-opening metathesis polymerization (ROMP). Dioctyloxy-substituted [2.2]metaparacyclophane-1,9-diene () (), was synthesized and fully characterized by IR, 1D-NMR, and 2D-NMR. A novel fully conjugated polymer, poly(m,p-phenylenevinylene), along with rod–coil copolymers () incorporating and non-conjugated units Norbornene Dicarboximides (NDI), was synthesized utilizing ROMP with second-generation Grubbs catalyst (G2). Kinetics studies examined using GPC confirmed controlled living polymerization in homopolymer and block designs, evidenced by PDI 1.10–1.17 and a close agreement between experimental and calculated Mn values. In contrast, random copolymers exhibited broader distributions due to ring strain mismatch. Optical measurements revealed tunable band gaps, Eopg 2.53–2.56 eV, and electrochemical gaps, Eelcg 2.01–2.37 eV, with enhanced conjugation in the homopolymer leading to narrower gaps. Morphological investigations using SEM showed distinct self-assembly behaviours influenced by the polymer chain and the micellization protocol, which explains the enhancement in the optical properties of these polymers with Eopg 2.27–2.41 eV. TGA analysis demonstrated high thermal stability across all polymers, with a range of around 368.2–394.6 °C. DFT and TD-DFT calculations confirmed that the effective conjugation length has been gained in all polymers. These findings highlight the versatility of ROMP in creating conjugated polymers with tunable optoelectronic performance and improved thermal stability, making them promising for flexible electronics applications.
聚(m,对苯乙烯)和功能化降冰片烯-二碳酰亚胺共聚物的活性ROMP:用DFT指导合成增强光电和热性能
通过开环复分解聚合(ROMP)合成了一系列功能化共聚物。合成了二辛基氧基取代的[2.2]偏对环己烷-1,9-二烯(DO-mp-CPDE) (M1),并通过IR、1D-NMR和2D-NMR进行了表征。在第二代Grubbs催化剂(G2)的催化下,利用ROMP合成了一种新型的全共轭聚合物聚(m,对苯基乙烯),以及含有DO-mp-CPDE和非共轭单元降冰片烯二酰亚胺(NDI)的棒圈共聚物(P2-P5)。使用GPC进行的动力学研究证实了均聚物和嵌段设计中可控的活性聚合,PDI为1.10-1.17,实验和计算的Mn值非常吻合。相比之下,无规共聚物由于环应变不匹配而表现出更广泛的分布。光学测量显示,可调谐的带隙E_g^op 2.53-2.56 eV和电化学间隙E_g^elc 2.01-2.37 eV,均聚物中增强的共轭作用导致更窄的带隙。SEM形态学研究表明,受聚合物链和胶束协议的影响,聚合物具有明显的自组装行为,这解释了E_g^op 2.27-2.41 eV对聚合物光学性能的增强。TGA分析表明,所有聚合物的热稳定性都很高,温度范围在368.2-394.6°C左右。DFT和TD-DFT计算证实了所有聚合物的有效共轭长度。这些发现突出了ROMP在创造具有可调光电性能和改善热稳定性的共轭聚合物方面的多功能性,使其在柔性电子应用中具有前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


