Identification of VVD-214/RO7589831, a Clinical-Stage, Covalent Allosteric Inhibitor of WRN Helicase for the Treatment of MSI-High Cancers
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Werner syndrome helicase (WRN) has emerged as a compelling therapeutic target for microsatellite instability-high (MSI-H) cancers, owing to its selective dependency on the helicase activity of WRN. Despite the inherent challenges in targeting helicases, our chemoproteomics approach enabled the identification of compounds that covalently engage C727 within an allosteric pocket of WRN, thereby inhibiting its ability to unwind DNA. Through optimization of each molecular component, particularly focusing on the vinyl sulfone warhead and C2 substitution at the pyrimidine core, an optimal balance of intrinsic reactivity, inhibitory potency, and metabolic stability was achieved, culminating in the identification of VVD-214/RO7589831. This process underscored the tunability of the vinyl sulfone warhead and its effectiveness in covalent drug discovery. VVD-214 induced tumor regression in MSI-H colorectal cancer models and is being evaluated as a promising therapeutic candidate for MSI-H cancers.
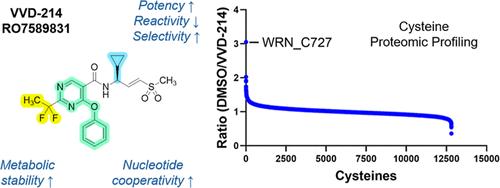
临床阶段WRN解旋酶共价变构抑制剂VVD-214/RO7589831治疗msi高肿瘤的鉴定
由于Werner综合征解旋酶(WRN)选择性依赖于WRN解旋酶活性,它已成为微卫星不稳定性高(MSI-H)癌症的一个令人信服的治疗靶点。尽管靶向解旋酶存在固有的挑战,但我们的化学蛋白质组学方法能够鉴定出在WRN变构口袋内共价结合C727的化合物,从而抑制其解开DNA的能力。通过对每个分子组分的优化,特别是对乙烯基砜战斗部和嘧啶核心的C2取代进行优化,实现了内在反应性、抑制效力和代谢稳定性的最佳平衡,最终鉴定出VVD-214/RO7589831。这一过程强调了乙烯基砜战斗部的可调性及其在共价药物发现中的有效性。VVD-214在MSI-H结直肠癌模型中诱导肿瘤消退,并被评估为MSI-H癌症的有希望的治疗候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


