Novel proline p-toluenesulfonate ionic liquid as a recyclable catalyst for lauric acid esterification: process optimisation, kinetic and thermodynamic studies
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this study, eight amino acid-based p-toluenesulfonic acid ionic liquids ([AAH][C7H7O3S]-ILs) were successfully prepared by the neutralisation reaction of natural amino acids with p-toluenesulfonic acid (p-TSA). The catalytic performance of the newly synthesized [AAH][C7H7O3S]-ILs was systematically investigated for biodiesel preparation using lauric acid and ethanol as model reactants. Experimental results demonstrated that proline p-toluenesulfonic acid ionic liquid ([ProH][C7H7O3S]) exhibited optimal catalytic performance for esterification of lauric acid and ethanol with a lower H0 (1.6601). Response surface methodology (RSM) optimized the process conditions to achieve 96.1 % lauric acid conversion under the following conditions: 14.4 wt.% catalyst loading, 16.3:1 ethanol/lauric acid molar ratio, 2.46 h reaction time, and 89 °C temperature. According to the 1H NMR method, the yield of biodiesel (ethyl laurate) was determined to be 96.0 %. The kinetic investigation revealed that the reaction followed first-order kinetic, exhibiting relatively low activation energy (7.04 kJ•mol−1) and a frequency factor reaching 0.208 min−1. Calculated thermodynamic parameters showed ΔH = 3.94 kJ•mol−1, ΔS = -0.268kJ•mol−1•K−1, ΔG = 103.9kJ•mol−1. After undergoing ten reaction cycles, the catalyst maintained 93.9 % of its initial catalytic activity, confirming its excellent structural stability. It was noteworthy that [ProH][C7H7O3S] exhibited homogeneous catalytic properties during the reaction, and the system can achieve spontaneous liquid–liquid partitioning after the reaction, which significantly simplifies the product separation process. The catalyst had the advantages of high catalytic efficiency, green renewability and easy operation, which provided a new catalytic system with the potential of industrialisation for biodiesel production.

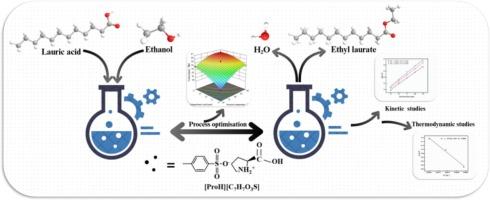
新型脯氨酸对甲苯磺酸离子液体作为月桂酸酯化的可回收催化剂:工艺优化、动力学和热力学研究
本研究通过天然氨基酸与对甲苯磺酸(p-TSA)的中和反应,成功制备了8种氨基酸基对甲苯磺酸离子液体([AAH][C7H7O3S]-ILs)。以月桂酸和乙醇为模型原料,系统研究了新合成的[AAH][C7H7O3S]- il在制备生物柴油中的催化性能。实验结果表明,脯氨酸对甲苯磺酸离子液体([ProH][C7H7O3S])在较低的H0(1.6601)下对月桂酸与乙醇的酯化反应表现出最佳的催化性能。响应面法优化的工艺条件为:催化剂负载14.4 wt%,乙醇/月桂酸摩尔比16.3:1,反应时间2.46 h,反应温度89 ℃,月桂酸转化率为96.1 %。经1H NMR法测定,月桂酸乙酯生物柴油的产率为96.0 %。动力学研究表明,该反应符合一级动力学,具有较低的活化能(7.04 kJ•mol−1)和频率因子(0.208 min−1)。计算热力学参数为ΔH = 3.94 kJ•mol−1,ΔS = -0.268kJ•mol−1•K−1,ΔG = 103.9kJ•mol−1。经过10个反应循环后,催化剂的初始催化活性仍保持在93.9 %,证实了其优异的结构稳定性。值得注意的是,[ProH][C7H7O3S]在反应过程中表现出均相的催化性能,反应后体系可以实现自发的液液分配,显著简化了产物分离过程。该催化剂具有催化效率高、绿色可再生、操作简便等优点,为生物柴油生产提供了一种具有工业化潜力的新型催化体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


