Fragment-Based Discovery and Structure-Led Optimization of MSC778, the First Potent, Selective, and Orally Bioavailable FEN1 Inhibitor
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
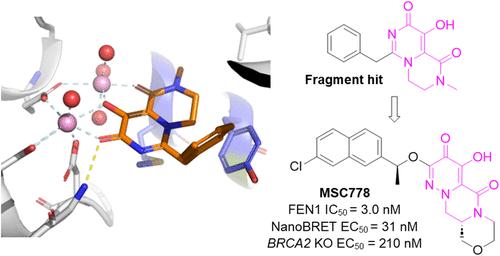
Flap endonuclease 1 (FEN1) is a long-standing target of interest in the DNA damage response (DDR) field due to its therapeutic potential in BRCA mutant cancers. To-date there have only been a handful of FEN1 inhibitors reported in the literature, most of which display modest selectivity and/or weak cellular activity. As such, there is a need for more advanced pharmacological tools to probe the biology of FEN1. Here, we report the discovery of MSC778, the first potent, selective, and orally bioavailable FEN1 inhibitor. We describe our metal-chelating fragment screening approach and structure-based optimization to identify MSC778, using structural insights to drive design. Consistent with FEN1 inhibition, MSC778 selectively kills BRCA2-deficient cells and potentiates the activity of PARPi niraparib in vivo to induce tumor stasis in a BRCA2 KO DLD-1 mouse xenograft. Furthermore, we illustrate how development of this approach has the potential for addressing nucleases as a target class.
基于片段的发现和结构导向优化MSC778,第一个有效的,选择性的,口服生物可利用的FEN1抑制剂
皮瓣内切酶1 (FEN1)是DNA损伤反应(DDR)领域长期关注的靶标,因为它在BRCA突变型癌症中的治疗潜力。迄今为止,文献中只报道了少数几种FEN1抑制剂,其中大多数表现出适度的选择性和/或弱细胞活性。因此,需要更先进的药理学工具来探索FEN1的生物学特性。在这里,我们报道了MSC778的发现,这是第一个有效的、选择性的、口服生物可利用的FEN1抑制剂。我们描述了我们的金属螯合碎片筛选方法和基于结构的优化来识别MSC778,使用结构见解来驱动设计。与FEN1抑制一致,MSC778选择性地杀死BRCA2缺陷细胞,并在体内增强PARPi尼拉帕尼的活性,以诱导BRCA2 KO dpd -1小鼠异种移植瘤的肿瘤停滞。此外,我们说明了这种方法的发展如何具有将核酸酶作为靶标类的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


