Fluorinated albumin nanocages dually target CAFs and tumor cells to potentiate bladder cancer chemoimmunotherapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Immunogenic cell death (ICD) induced by chemotherapeutics holds promise for cancer therapy, but limited drug penetration across the bladder mucosa and an immune-excluded tumor microenvironment (TME) have hindered success in bladder cancer. Here, we develop fluorinated albumin nanocages as transmucosal delivery vesicles that concurrently target cancer-associated fibroblasts (CAFs) and tumor cells to potentiate chemoimmunotherapy. Surface fluorination enables mucosal penetration, while recognition of secreted protein acidic and cysteine-rich (SPARC) protein ensures selective uptake by CAFs and tumor cells. Encapsulated chemotherapeutics enhance ICD through inhibition of the Bcl-2 pathway, promoting tumor cell death. Simultaneous CAF disruption reduces stromal fibrosis, facilitating anti–PD-L1 antibody delivery and T-cell infiltration. This dual-targeting strategy synergizes ICD with immune checkpoint blockade to eradicate bladder tumors by recruiting cytotoxic T cells and suppressing immunosuppressive phenotypes. Ex vivo studies in freshly resected human bladder tumors further validated the translational potential. Our findings highlight the fluorinated albumin nanocages as a versatile transmucosal platform to remodel the tumor–stroma axis and amplify chemoimmunotherapy in bladder cancer.
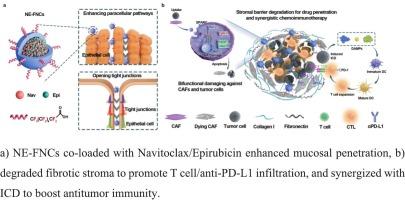
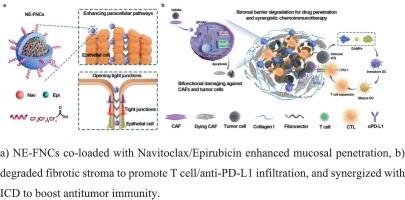
氟化白蛋白纳米笼双重靶向caf和肿瘤细胞,以增强膀胱癌的化学免疫治疗
化疗药物诱导的免疫原性细胞死亡(ICD)为癌症治疗带来了希望,但药物穿过膀胱粘膜的有限渗透和免疫排斥肿瘤微环境(TME)阻碍了膀胱癌治疗的成功。在这里,我们开发了氟化白蛋白纳米笼作为跨粘膜递送囊泡,同时靶向癌症相关成纤维细胞(CAFs)和肿瘤细胞,以增强化学免疫治疗。表面氟化使粘膜渗透,而识别分泌的蛋白质酸性和富含半胱氨酸(SPARC)蛋白,确保CAFs和肿瘤细胞选择性摄取。包封化疗药物通过抑制Bcl-2通路,促进肿瘤细胞死亡,从而增强ICD。同时CAF的破坏减少了间质纤维化,促进了抗pd - l1抗体的传递和t细胞的浸润。这种双重靶向策略通过募集细胞毒性T细胞和抑制免疫抑制表型,使ICD与免疫检查点阻断协同消除膀胱肿瘤。对刚切除的人类膀胱肿瘤的体外研究进一步证实了这种方法的翻译潜力。我们的研究结果强调了氟化白蛋白纳米笼作为一种通用的跨粘膜平台,可以重塑肿瘤-基质轴并增强膀胱癌的化学免疫治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


