Electrochemical Carboxylative Cyclization of Propargylamines with CO2 to Functionalized 2-Oxazolidinones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The electrochemical conversion of CO2 into value-added chemicals represents a promising strategy for sustainable synthesis. Herein, we report a novel electrochemical carboxylative cyclization of propargylamines with CO2 to access exocyclic iodo-carboxyl-vinylene 2-oxazolidinones bearing two CO2 moieties by using tetraethylammonium iodide (Et4NI) as both the electrolyte and the “iodine source”, which enables the one-pot construction of C–O, C–C, C–N, and C–I bonds. The protocol proceeds under mild conditions and accommodates a broad range of substrates, providing access to functionalized 2-oxazolidinones with high structural complexity.

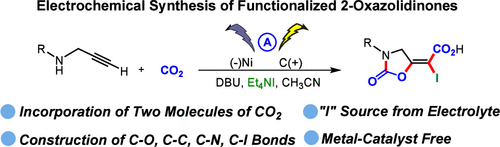
丙胺与CO2的电化学羧化环化制备2-恶唑烷酮
电化学将二氧化碳转化为增值化学品是一种有前途的可持续合成策略。在此,我们报道了一种新的丙胺与CO2的电化学羧化环化,通过使用四乙基碘化铵(Et4NI)作为电解质和“碘源”,获得含有两个CO2基团的外环碘-羧基-乙烯- 2-恶唑烷酮,从而实现了C-O, C-C, C-N和C-I键的一次构建。该工艺在温和的条件下进行,适应广泛的底物,提供了具有高结构复杂性的功能化2-恶唑烷酮。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


