SAR studies of chabrolonaphthoquinone B via rational design and synthesis of analogues - Cytotoxicity evaluation uncovers anticancer activity via mRNA splicing modulation
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A series of meroterpenoid cytotoxic natural product chabrolonaphthoquinone B (1) analogues were rationally designed. Key structural modifications of the parent compound were introduced to elucidate the features essential for optimal biological activity. Versatile and efficient synthetic strategies were employed to access the designed analogues. The SAR studies revealed that the quinone ring system in 1 is the key structural feature responsible for the observed cytotoxicity while other groups also contribute to the activity against a broad panel of cancer cell lines. Further evaluation of 1 and selected active analogues demonstrated their ability to modulate the mRNA splicing process in HeLa cells, a mode of cytotoxic activity that has only been sporadically attributed to the quinone moiety in previous studies.
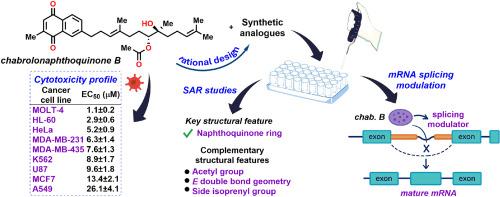
通过合理设计和合成类似物研究chbrolonaphthoquinone B的SAR -细胞毒性评价揭示了mRNA剪接调节的抗癌活性
合理设计了一系列巯基萜类细胞毒性天然产物恰溴萘醌B(1)类似物。介绍了母体化合物的关键结构修饰,以阐明优化生物活性所必需的特征。采用灵活高效的合成策略获得设计的类似物。SAR研究表明,1中的醌环系统是负责观察到的细胞毒性的关键结构特征,而其他组也有助于对抗广泛的癌细胞系的活性。对1和选定的活性类似物的进一步评估表明,它们能够调节HeLa细胞中的mRNA剪接过程,这是一种细胞毒性活性模式,在先前的研究中仅零星地归因于醌部分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


