Highly active and durable MNS bifunctional electrocatalysts for enhanced anion exchange membrane water electrolysis
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
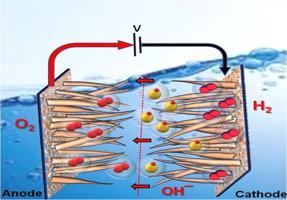
The development of non-platinum group metal (non-PGM) electrocatalysts with performance comparable to their noble metal counterparts remains a significant challenge for overall water splitting. In this study, we demonstrate the performance of an Anion Exchange Membrane (AEM) water electrolyzer using a bifunctional, non-PGM electrocatalyst: reduced graphene oxide (rGO)-encapsulated MoS2/Ni3S2 (MNS) grown on a nickel foam (NF) substrate. The rGO/MoS2/Ni3S2 (rGO-MNS) electrode was synthesized via a facile, single-step hydrothermal method. For the hydrogen evolution reaction (HER), the rGO-MNS electrode exhibited a low overpotential of 94 mV at a current density of 100 mA cm⁻², maintaining excellent stability over 50 h with a minimal degradation rate of 120 µV h⁻¹. In the case of the oxygen evolution reaction (OER), an overpotential of 410 mV was required to reach the same current density, with a similarly robust durability and a degradation rate of only 360 µV h⁻¹. When employed as symmetric electrodes for overall water electrolysis, the rGO-MNS system achieved a current density of 10 mA cm⁻² at a cell voltage of 1.51 V, outperforming the benchmark Pt/C‖Ru/C catalyst pair, which required 1.58 V to reach the same performance. The enhanced electrocatalytic activity and durability are attributed to the conductive rGO encapsulation, which facilitates charge transfer and mitigates surface oxidation of the catalyst. These results present a promising strategy for designing cost-effective, durable, and high-performance non-PGM electrodes for AEM water electrolysis.
增强型阴离子交换膜电解用高效耐用MNS双功能电催化剂
开发性能可与贵金属相媲美的非铂族金属电催化剂仍然是整体水分解的重大挑战。在这项研究中,我们展示了阴离子交换膜(AEM)水电解槽的性能,该电解槽使用双功能、非pgm电催化剂:在泡沫镍(NF)衬底上生长的还原氧化石墨烯(rGO)封装的MoS2/Ni3S2 (MNS)。采用简单的单步水热法合成了rGO/MoS2/Ni3S2 (rGO- mns)电极。对于析氢反应(HER), rGO-MNS电极在100 mA cm⁻²电流密度下表现出94 mV的低过电位,在50小时内保持良好的稳定性,最小的降解率为120µV h⁻¹。在析氧反应(OER)的情况下,需要410 mV的过电位才能达到相同的电流密度,具有类似的坚固耐用性,降解率仅为360µV h⁻¹。当采用rGO-MNS系统作为整体水电解的对称电极时,在1.51 V的电池电压下,rGO-MNS系统实现了10 mA cm⁻²的电流密度,优于基准Pt/C‖Ru/C催化剂对,后者需要1.58 V才能达到相同的性能。增强的电催化活性和耐用性归功于导电氧化石墨烯的封装,这有助于电荷转移并减轻催化剂的表面氧化。这些结果为AEM水电解设计具有成本效益、耐用性和高性能的非pgm电极提供了一个有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


