Fungal resistance in rice is restored by interfamily transfer of an evolutionarily lost co-receptor
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
The interfamily transfer of pattern-recognition receptors (PRRs) offers a promising strategy to enhance plant immunity; however, factors causing functional limitations across species remain unknown. Here, we identify secreted TOM20 domain-containing protein (STOM), a previously uncharacterized fungal microbe-associated molecular pattern (MAMP) that triggers immunity in Nicotiana benthamiana but not in rice (Oryza sativa). We identify NbSTOMR as the receptor that recognizes and binds STOM, and NbSTOMRh as the co-receptor that, despite lacking ligand-binding ability, is essential through its extracellular interaction with NbSTOMR. Transferring NbSTOMR to rice fails to confer resistance, but NbSTOMRh alone enhances resistance to false smut and blast disease. Evolutionary analyses reveal that while STOMR is conserved, monocots have lost STOMRh due to transposon-mediated chromosomal separation of its extracellular domain. Although OsSTOMR binds STOM, OsSTOMRh is non-functional; however, NbSTOMRh promotes OsSTOMR-dependent STOM recognition. These findings highlight the critical role of co-receptors in overcoming taxonomic barriers and provide a strategy for reconstituting PRR-mediated immunity in monocot crops.
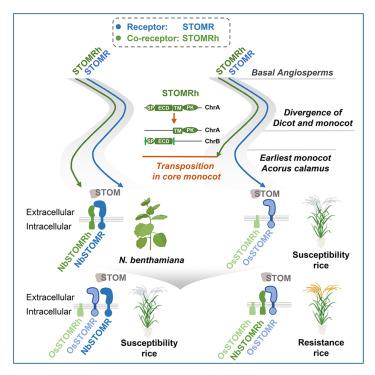
水稻的真菌抗性是通过一种进化上丢失的共受体的家族间转移而恢复的
模式识别受体(PRRs)的家族间转移为增强植物免疫提供了一种有前景的策略;然而,造成跨物种功能限制的因素仍然未知。在这里,我们鉴定了分泌的含有TOM20结构域的蛋白(STOM),这是一种以前未被表征的真菌微生物相关分子模式(MAMP),它在烟叶(Nicotiana benthamiana)中触发免疫,但在水稻(Oryza sativa)中没有。我们确定NbSTOMR是识别和结合STOM的受体,NbSTOMRh是协同受体,尽管缺乏配体结合能力,但通过与NbSTOMR的细胞外相互作用是必不可少的。将NbSTOMR转移到水稻中不能产生抗性,但NbSTOMRh单独增强对假黑穗病和稻瘟病的抗性。进化分析表明,虽然STOMR是保守的,但单子体由于转座子介导的细胞外结构域的染色体分离而失去了STOMRh。虽然OsSTOMR与STOM结合,但OsSTOMRh是无功能的;然而,NbSTOMRh促进依赖于osstomr的stomr识别。这些发现突出了共受体在克服分类障碍方面的关键作用,并为在单子叶作物中重建prr介导的免疫提供了策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


