Oral multifunctional gel beads for the targeted treatment of inflammatory bowel disease
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Current IBD therapies face limitations such as systemic toxicity, off-target effects, and microbiota dysbiosis. We developed an oral multifunctional gel bead integrating three innovations: (1) a butyrate–apigenin synergy that inhibits NF-κB via CK2/p65 phosphorylation suppression, activates a PPAR-γ-mediated metabolic shift to β-oxidation, and modulates the microbiota by enhancing Bacteroidetes/suppressing Enterobacteriaceae; (2) an apigenin–butyrate ester prodrug overcoming organoleptic issues while enabling CK2 targeting; and (3) a pH/enzyme–responsive delivery system using the FDA-approved ascorbyl palmitate/alginate, achieving gastric protection, charge–mediated colitis targeting, and esterase–triggered release. In colitis model mice, gel beads restore clinical parameters, intestinal integrity, and microbial balance, outperforming apigenin-butyrate ester and 5-ASA at half-doses with extended intervals. It significantly downregulated colon CK2 expression (4.07-fold) and the phospho-P65/total P65 ratio (3.04-fold). This integrated strategy combining multimodal drug actions and precision delivery establishes a new paradigm for efficient and alternative IBD therapeutics with reduced dosing requirements.

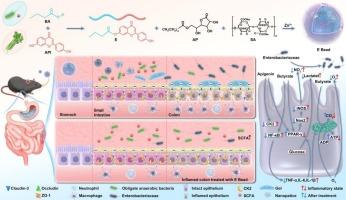
靶向治疗炎症性肠病的口服多功能凝胶珠
目前的IBD治疗面临局限性,如全身毒性、脱靶效应和微生物群失调。我们开发了一种口服多功能凝胶珠,整合了三个创新:(1)丁酸盐-芹菜素协同作用,通过抑制CK2/p65磷酸化抑制NF-κB,激活PPAR-γ介导的代谢向β-氧化的转变,并通过增强拟杆菌门/抑制肠杆菌科调节微生物群;(2)芹菜素-丁酸酯前药克服感官问题,同时使CK2靶向;(3)使用fda批准的抗坏血酸棕榈酸酯/海藻酸酯的pH/酶响应递送系统,实现胃保护、电荷介导的结肠炎靶向和酯酶触发释放。在结肠炎模型小鼠中,凝胶珠可恢复临床参数、肠道完整性和微生物平衡,其效果优于半剂量、延长间隔的芹菜素-丁酸酯和5-ASA。显著下调结肠CK2表达(4.07倍)和磷酸化-P65/总P65比值(3.04倍)。这种综合策略结合了多模式药物作用和精确给药,为减少剂量要求的有效和替代IBD治疗建立了新的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


