Assessing the anti-leukemic efficacy of a curcuminoid in cellular and animal models
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
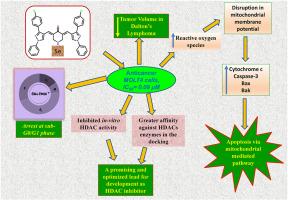
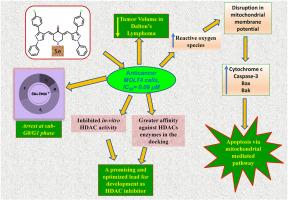
Leukemia ranks as the tenth most common cause of cancer-related deaths, and significant side effects often limit current treatments. Curcumin, a lipophilic polyphenol, has garnered considerable attention for its therapeutic potential in addressing various conditions, including inflammation, diabetes, aging, and cancer, particularly leukemia. In this study, ten novel curcuminoids were synthesized and structurally characterized for integrity and purity using spectral techniques. Cytotoxic evaluation against the MOLT-4, HEK, EL4, YAC1 and L1210 cell lines identified one compound, (3E,5E)-3,5-bis((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)piperidin-4-one (5e), to be potent, demonstrating superior cytotoxic activity compared to curcumin. Mechanistic studies revealed that treatment with 5e induced the production of reactive oxygen species (ROS), disrupted mitochondrial membrane potential, and promoted apoptosis, as confirmed by annexin-V-FITC/PI staining. Cell cycle analysis further showed significant accumulation in the sub-G0/G1 phase, consistent with apoptotic or necrotic cell death. In vivo studies using Dalton's Lymphoma models demonstrated that 5e effectively reduced tumor volume with minimal systemic toxicity. Western blot analyses confirmed activation of the intrinsic apoptotic pathway, with significant upregulation of cytochrome c and cleaved caspase-3. Moreover, 5e inhibited HDACs significantly in vitro, with activity greater than curcumin and comparable to trichostatin A, suggesting a target of anticancer action. Additionally, molecular docking studies suggested that 5e acts as a potential HDAC8 inhibitor, correlating with its enhanced anticancer activity. Collectively, these findings establish compound 5e as a promising curcuminoid analogue with potent cytotoxic and anti-proliferative effects against leukemia, highlighting its therapeutic potential.
在细胞和动物模型中评估姜黄素类抗白血病功效
白血病是癌症相关死亡的第十大常见原因,其严重的副作用往往限制了目前的治疗。姜黄素是一种亲脂性多酚,因其治疗各种疾病的潜力而受到广泛关注,包括炎症、糖尿病、衰老和癌症,特别是白血病。本研究合成了10种新的姜黄素类化合物,并利用光谱技术对其完整性和纯度进行了结构表征。对MOLT-4、HEK、EL4、YAC1和L1210细胞系进行细胞毒性评价,鉴定出一种化合物(3E,5E)-3,5-二((3-(4-氯苯基)-1-苯基- 1h -吡唑-4-基)亚甲基)胡椒苷-4-one (5E)具有较强的细胞毒性,与姜黄素相比具有更强的细胞毒性。机制研究表明,经annexin-V-FITC/PI染色证实,5e可诱导活性氧(ROS)的产生,破坏线粒体膜电位,促进细胞凋亡。细胞周期分析进一步显示,在亚g0 /G1期有明显的积累,与细胞凋亡或坏死死亡一致。使用道尔顿淋巴瘤模型进行的体内研究表明,5e有效地减少了肿瘤体积,且系统毒性最小。Western blot分析证实了内在凋亡通路的激活,细胞色素c和cleaved caspase-3显著上调。此外,5e在体外显著抑制hdac,其活性大于姜黄素,与曲古霉素A相当,提示其具有抗癌作用的靶点。此外,分子对接研究表明,5e作为一种潜在的HDAC8抑制剂,与其增强的抗癌活性相关。总的来说,这些发现表明化合物5e是一种有前途的姜黄素类似物,对白血病具有有效的细胞毒性和抗增殖作用,突出了其治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


