Access to chiral spiroketals via catalytic enantioselective halogenation of racemic olefinic hemiketals
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chiral spiroketals are privileged structural motifs that widely appear in natural products, pharmaceutical agents, and chiral catalysts. Nevertheless, catalytic asymmetric methods for synthesizing chiral spiroketals remain scarce. Here, we report an asymmetric catalytic halogenation of racemic olefinic hemiketals to synthesize chiral halo-spiroketals. The approach utilizes a cross-assembled bifunctional catalyst system that integrates a chiral phosphoric acid with an achiral quinoline base. Optimization of the reaction was accomplished mainly by modifying the cost-effective achiral quinoline. The reaction mechanism is characterized by a dynamic kinetic resolution of hemiketal and a diastereoselective bromocyclization, underscoring the critical function of the achiral quinoline base throughout both phases of the catalytic process. The chiral halo-spiroketals are important intermediates for the synthesis of chiral spiroketal phosphine ligands, which can be applied in various asymmetric catalytic reactions. The halogen substituents in the spiroketal phosphine ligands enable late-stage modifications that aid in the selection of suitable ligands for particular reactions.
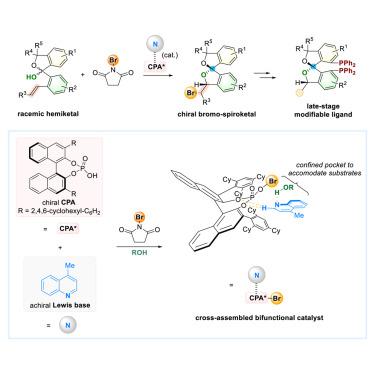
外消旋烯烃半酮催化对映选择性卤化制备手性螺旋酮
手性螺旋酮是一种特殊的结构基序,广泛存在于天然产物、药物制剂和手性催化剂中。然而,合成手性螺旋酮的催化不对称方法仍然很少。本文报道了外消旋烯烃半酮的不对称催化卤化反应,合成手性环旋酮。该方法利用交叉组装的双功能催化剂系统,将手性磷酸与非手性喹啉碱结合在一起。对反应的优化主要通过对低成本的非手性喹啉进行改性来完成。该反应机制的特点是半晶体和非对映选择性溴环化的动态动力学分解,强调了非手性喹啉碱在催化过程中两个阶段的关键作用。手性环旋酮是合成手性旋酮膦配体的重要中间体,可用于各种不对称催化反应。螺旋酮膦配体中的卤素取代基可以进行后期修饰,有助于为特定反应选择合适的配体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


